Exogenous and Endogeneous Disialosyl Ganglioside GD1b Induces Apoptosis of MCF-7 Human Breast Cancer Cells
Abstract
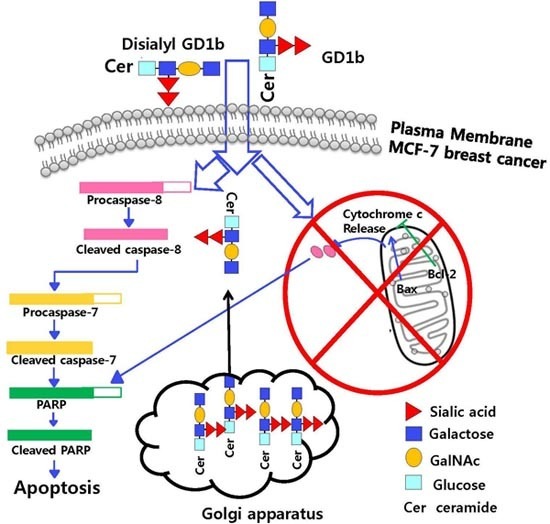
:1. Introduction
2. Results
2.1. Suppression of Cell Growth by GD1b
2.2. Induction of Apoptosis by GD1b in MCF-7 Cells
2.3. Induction of Apoptosis Related-Molecules by GD1b
2.4. Expression of Gangliosides by Transfection of GD1b Synthase
2.5. Suppression of Cell Growth by GD1b Overexpression
2.6. Induction of Apoptosis Related Molecules by GD1b Overexpression
3. Discussion
4. Material and Methods
4.1. Cells and Materials
4.2. XTT Proliferation Assay
4.3. Flow Cytometry Assay
4.4. Immunofluorescence
4.5. Immunoblot Analysis
4.6. Molecular Cloning of the Human β1,3-Galactosyltransferase-2 Gene (GM1 and GD1b Synthase; Gal-T2) and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
4.7. Gene Transfection and Selection
4.8. Extraction of Glycolipids and HPTLC
4.9. Data Analysis
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fenderson, B.A.; Eddy, E.M.; Hakomori, S. Glycoconjugate expression during embryogenesis and its biological significance. Bioessays 1990, 12, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Wada, T.; Iwamatsu, A.; Hata, K.; Yoshikawa, Y.; Tokuyama, S.; Sawada, M. Molecular cloning and characterization of a plasma membrane-associated sialidase specific for gangliosides. J. Biol. Chem. 1999, 274, 5004–5011. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chi, S.; Liu, M.; Yang, W.; Wei, T.; Qi, Z.; Yang, F. Inhibitory effect of ganglioside GD1b on K+ current in hippocampal neurons and its involvement in apoptosis suppression. J. Lipid Res. 2005, 46, 2580–2585. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Chung, T.W.; Kang, S.K.; Lee, Y.C.; Ko, J.H.; Kim, J.G.; Kim, C.H. Ganglioside GM3 modulates tumor suppressor PTEN-mediated cell cycle progression—Transcriptional induction of p21(WAF1) and p27(kip1) by inhibition of PI-3K/AKT pathway. Glycobiology 2006, 16, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.Y.; Kang, S.K.; Lee, Y.C.; Choi, H.J.; Lee, Y.S.; Cho, S.Y.; Kim, Y.S.; Ko, J.H.; Kim, C.H. Transcriptional regulation of the human GD3 synthase gene expression in Fas-induced Jurkat T cells: A critical role of transcription factor NF-kappaB in regulated expression. Glycobiology 2006, 16, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.; Kim, Y.S.; Kim, H.T.; Kim, C.H.; Cho, E.W.; Kang, H.Y.; Kim, N.S.; Kim, C.H.; Ryu, S.E.; Lee, J.H.; et al. Ganglioside GM3 is involved in neuronal cell death. Fed. Am. Soc. Exp. Biol. J. 2006, 20, 1248–1250. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.T.; Lee, Y.C.; Kim, C.H. Overexpression of GD3 synthase induces apoptosis of vascular endothelial ECV304 cells through downregulation of Bcl-2. FEBS Lett. 2004, 568, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.K.; Kim, H.M.; Lee, Y.C.; Kim, C.H. Disialoganglioside (GD3) synthase gene expression suppresses vascular smooth muscle cell responses via the inhibition of ERK1/2 phosphorylation, cell cycle progression, and matrix metalloproteinase-9 expression. J. Biol. Chem. 2004, 279, 33063–33070. [Google Scholar] [CrossRef] [PubMed]
- Paris, R.; Morales, A.; Coll, O.; Sanchez-Reyes, A.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Ganglioside GD3 sensitizes human hepatoma cells to cancer therapy. J. Biol. Chem. 2002, 277, 49870–49876. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ma, R.; Boyle, P.J.; Mikulla, B.; Bradley, M.; Smith, B.; Basu, M.; Banerjee, S. Apoptosis of human carcinoma cells in the presence of potential anti-cancer drugs: III. Treatment of Colo-205 and SKBR3 cells with: cis-platin, Tamoxifen, Melphalan, Betulinic acid, L-PDMP, L-PPMP, and GD3 ganglioside. Glycoconj. J. 2004, 20, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Fukumoto, S.; Kawaguchi, H.; Sato, S.; Ueda, R.; Furukawa, K. Ganglioside G(D2) in small cell lung cancer cell lines: Enhancement of cell proliferation and mediation of apoptosis. Cancer Res. 2001, 61, 4244–4252. [Google Scholar] [PubMed]
- Ma, R.; Koulov, A.; Moulton, C.; Basu, M.; Banerjee, S.; Goodson, H.; Basu, S. Apoptosis of human breast carcinoma cells in the presence of disialosyl gangliosides: II. Treatment of SKBR3 cells with GD3 and GD1b gangliosides. Glycoconj. J. 2004, 20, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Evans, V.G. Multiple pathways to apoptosis. Cell Biol. Int. 1993, 17, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Moon, D.O.; Rhu, C.H.; Choi, B.T.; Lee, W.H.; Kim, G.Y.; Choi, Y.H. β-Sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol. Pharm. Bull. 2007, 30, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kwon, H.Y.; Lee, E.O.; Lee, H.J.; Ahn, K.S.; Kim, M.O.; Kim, C.H.; Ahn, K.S.; Kim, S.H. DMNQ-S17 inhibits constitutive NF-kappaB activation leading to induction of apoptosis through the activation of caspase-3 in human myeloid leukemia U937 cells. Life Sci. 2008, 83, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Liu, Y.Q.; Cui, Y.F. Pathways to caspase activation. Cell Biol. Int. 2005, 29, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Antonsson, B. Mitochondria and the Bcl-2 family proteins in apoptosis signaling pathways. Mol. Cell Biochem. 2004, 256–257, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, E.C.; Medema, J.P. Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat. Rev. 2008, 34, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banerjee, S.; Wang, Z.; Kong, D.; Sarkar, F.H. Plumbagin-Induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J. Cell. Biochem. 2008, 105, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Goodison, S.; Viars, C.; Grazzini, M.; Urquidi, V. The interrelationship between DRIM gene expression and cytogenetic and phenotypic characteristics in human breast tumor cell lines. BMC Genom. 2003, 4, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, M.U.; Naik, T.U.; Suckow, A.T.; Duncan, M.K.; Naik, U.P. Attenuation of junctional adhesion molecule-A is a contributing factor for breast cancer cell invasion. Cancer Res. 2008, 68, 2194–2203. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.; Malone, C.; Chumsri, S.; Burger, A.M.; McDonnell, S. Characterisation of breast cancer cell lines and establishment of a novel isogenic subclone to study migration, invasion and tumourigenicity. Clin. Exp. Metastasis 2008, 25, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Wang, F.; Spiegel, S. Glycosphingolipid composition of MDA-MB-231 and MCF-7 human breast cancer cell lines. Breast Cancer Res. Treat. 1998, 48, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Parvinen, M. Stage-Specific apoptosis of male germ cells in the rat: Mechanisms of cell death studied by supravital squash preparations. Tissue Cell 1998, 30, 692–701. [Google Scholar] [CrossRef]
- Jin, U.H.; Song, K.H.; Motomura, M.; Suzuki, I.; Gu, Y.H.; Kang, Y.J.; Moon, T.C.; Kim, C.H. Caffeic acid phenethyl ester induces mitochondria-mediated apoptosis in human myeloid leukemia U937 cells. Mol. Cell. Biochem. 2008, 310, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K. Role of mitochondria as the gardens of cell death. Cancer Chemother. Pharmacol. 2006, 57, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yuan, J. Cross-Talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J. Cell Biol. 2000, 150, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Inoki, Y.; Miura, T.; Kajimoto, T.; Kawase, M.; Kawase, Y.; Yoshida, Y.; Tsuji, S.; Kinouchi, T.; Endo, H.; Kagawa, Y.; et al. Ganglioside GD3 and its mimetics induce cytochrome c release from mitochondria. Biochem. Biophys. Res. Commun. 2000, 276, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Mutoh, T.; Hasegawa, T.; Miyazaki, H.; Okada, M.; Goto, G.; Furukawa, K.; Urano, T. GD3 synthase gene expression in PC12 cells results in the continuous activation of TrkA and ERK1/2 and enhanced proliferation. J. Biol. Chem. 2000, 275, 5832–5838. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J. Biol. Chem. 1990, 265, 18713–18716. [Google Scholar] [PubMed]
- Mocchetti, I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell. Mol. Life Sci. 2005, 62, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Chung, T.W.; Kim, S.J.; Cho, S.Y.; Lee, Y.S.; Lee, Y.C.; Ko, J.H.; Kim, C.H. The AP-2α transcription factor is required for the ganglioside GM3-stimulated transcriptional regulation of a PTEN gene. Glycobiology 2008, 18, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Sala, G.; Minutolo, F.; Macchia, M.; Sacchi, N.; Ghidoni, R. Resveratrol structure and ceramide-associated growth inhibition in prostate cancer cells. Drugs Exp. Clin. Res. 2003, 29, 263–269. [Google Scholar] [PubMed]
- Pink, J.J.; Wuerzberger-Davis, S.; Tagliarino, C.; Planchon, S.M.; Yang, X.; Froelich, C.J.; Boothman, D.A. Activation of a cysteine protease in MCF-7 and T47D breast cancer cells during beta-lapachone-mediated apoptosis. Exp. Cell Res. 2000, 255, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Stennicke, H.R.; Wang, B.; Green, D.R.; Janicke, R.U.; Srinivasan, A.; Seth, P.; Salvesen, G.S.; Froelich, C.J. Granzyme B mimics apical caspases. Description of a unified pathway for trans-activation of executioner caspase-3 and -7. J. Biol. Chem. 1998, 273, 34278–34283. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.L.; Wang, X.F.; Zhu, X.; Zhou, X.D.; Xu, L.H. Morphological and Biochemical Changes Associated with Apoptosis Induced by Okadaic Acid in Human Amniotic FL Cells. Environ. Toxicol. 2008, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidou, A.E.; Korkolopoulou, P.; Patsouris, E. Apoptotic markers for primary brain tumor prognosis. J. Neuro-Oncol. 2005, 72, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Jin, U.H.; Kim, K.W.; Lee, Y.C.; Park, Y.G.; Kim, C.H. Disialoganglioside GD3 increases in the secretion of apoB-containing lipoproteins. Biochem. Biophys. Res. Commun. 2007, 356, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Kato, K.; Yanagisawa, K. Abeta polymerization through interaction with membrane gangliosides. Biochim. Biophys. Acta 2010, 1801, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Majewski, J.; André, S.; Jones, E.; Chi, E.; Gabius, H.J. X-ray reflectivity and grazing incidence diffraction studies of interaction between human adhesion/growth-regulatory galectin-1 and DPPE-GM1 lipid monolayer at an air/water interface. Biochemistry 2015, 80, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Rosette, C.; Roth, R.B.; Oeth, P.; Braun, A.; Kammerer, S.; Ekblom, J.; Denissenko, M.F. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis 2005, 26, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Lee, G.W.; Lee, J.S.; Cho, M.K.; Son, K.H.; Jeon, S.J.; Kang, S.S.; Kim, Y.S.; Lee, J.H.; Seo, H.G.; et al. Tanshinone I suppresses growth and invasion of human breast cancer cells, MDA-MB-231, through regulation of adhesion molecules. Carcinogenesis 2008, 29, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Scambia, G.; Ranelletti, F.O.; Panici, P.B.; Piantelli, M.; De Vincenzo, R.; Ferrandina, G.; Bonanno, G.; Capelli, A.; Mancuso, S. Quercetin induces type-II estrogen-binding sites in estrogen-receptor-negative (MDA-MB231) and estrogen-receptor-positive (MCF-7) human breast-cancer cell lines. Int. J. Cancer 1993, 54, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Cazet, A.; Groux-Degroote, S.; Teylaert, B.; Kwon, K.M.; Lehoux, S.; Slomianny, C.; Kim, C.H.; Le Bourhis, X.; Delannoy, P. GD3 synthase overexpression enhances proliferation and migration of MDA-MB-231 breast cancer cells. Biol. Chem. 2009, 390, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Koo, D.B.; Ko, K.; Ko, K.; Kim, S.M.; Jung, J.U.; Ryu, J.S.; Jin, J.W.; Yang, H.J.; Do, S.I.; et al. Effects of daunorubicin on ganglioside expression and neuronal differentiation of mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2007, 362, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Aixinjueluo, W.; Kasama, T.; Ohkawa, Y.; Yoshihara, M.; Ohmi, Y.; Tajima, O.; Suzumura, A.; Kittaka, D.; Furukawa, K. Disruption of GM2/GD2 synthase gene resulted in over-expression of 9-O-acetyl GD3 irrespective of Tis21. J. Neurochem. 2008, 105, 1057–1066. [Google Scholar] [CrossRef] [PubMed]









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.-H.; Lee, J.-M.; Kwon, K.-M.; Kwak, C.-H.; Abekura, F.; Park, J.-Y.; Cho, S.-H.; Lee, K.; Chang, Y.-C.; Lee, Y.-C.; et al. Exogenous and Endogeneous Disialosyl Ganglioside GD1b Induces Apoptosis of MCF-7 Human Breast Cancer Cells. Int. J. Mol. Sci. 2016, 17, 652. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050652
Ha S-H, Lee J-M, Kwon K-M, Kwak C-H, Abekura F, Park J-Y, Cho S-H, Lee K, Chang Y-C, Lee Y-C, et al. Exogenous and Endogeneous Disialosyl Ganglioside GD1b Induces Apoptosis of MCF-7 Human Breast Cancer Cells. International Journal of Molecular Sciences. 2016; 17(5):652. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050652
Chicago/Turabian StyleHa, Sun-Hyung, Ji-Min Lee, Kyung-Min Kwon, Choong-Hwan Kwak, Fukushi Abekura, Jun-Young Park, Seung-Hak Cho, Kichoon Lee, Young-Chae Chang, Young-Choon Lee, and et al. 2016. "Exogenous and Endogeneous Disialosyl Ganglioside GD1b Induces Apoptosis of MCF-7 Human Breast Cancer Cells" International Journal of Molecular Sciences 17, no. 5: 652. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17050652