Prolonged Starvation Causes Up-Regulation of AQP1 in Adipose Tissue Capillaries of AQP7 Knock-Out Mice
Abstract
:1. Introduction
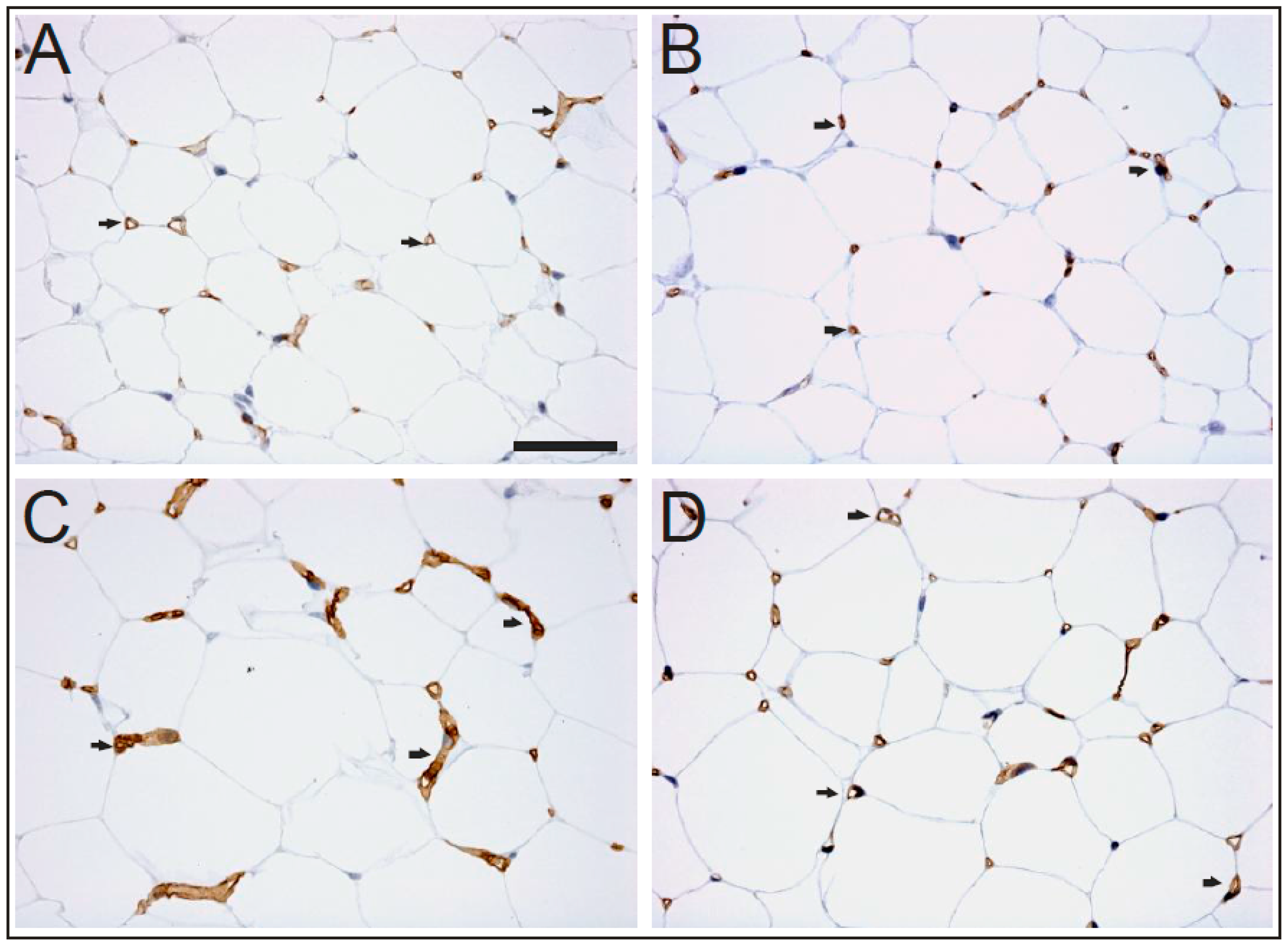
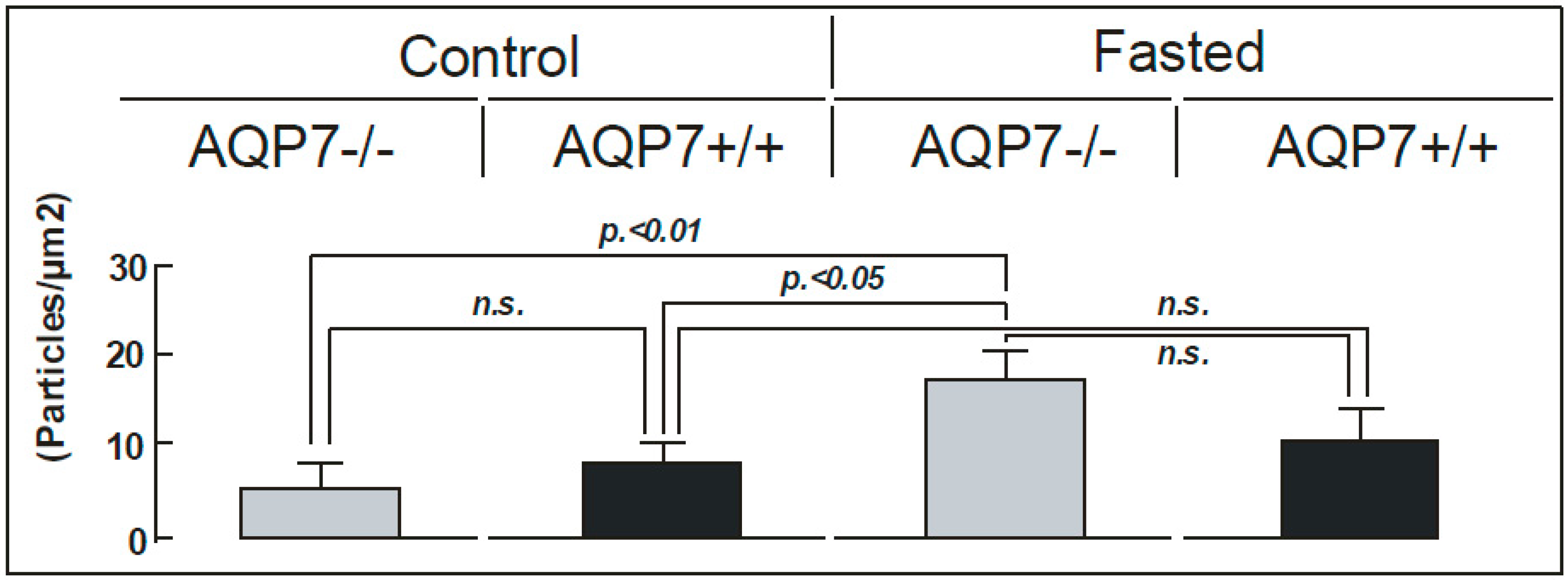
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Models
4.2. SDS-PAGE and Immunoblotting
4.3. Immunohistochemistry for Light Microscopic Examination
4.4. Immunoelectron Microscopy of Mouse Capillaries
4.5. Plasma/Urine Glycerol Measurements
4.6. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQPs | Aquaporins |
| AQP7 KO | AQP7 knock-out mice |
| cAMP | Cyclic adenosine monophosphate |
| Dot1l | Dot1l histone H3K79 methyltransferase |
| n.s. | non significant |
| RER | Rough endoplasmic reticulum |
| WAT | White adipose tissue |
References
- Rojek, A.; Praetorius, J.; Frøkiaer, J.; Nielsen, S.; Fenton, R.A. A current view of the mammalian aquaglyceroporins. Annu. Rev. Physiol. 2008, 70, 301–327. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Moura, T.F.; Soveral, G. Aquaglyceroporins: Implications in adipose biology and obesity. Cell. Mol. Life Sci. 2015, 72, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Mósca, A.F.; Moura, T.F.; Soveral, G. Aquaporin-5 is expressed in adipocytes with implications in adipose differentiation. IUBMB Life 2015, 67, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Lebeck, J.; Rojek, A.; Praetorius, J.; Füchtbauer, E.M.; Frøkiaer, J.; Nielsen, S. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: Implications in glycerol metabolism. Am. J. Physiol. Ren. Physiol. 2007, 292, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Benga, G. The first discavered water channel protein, later called aquaporin 1: Molecular characteristics, functions and medical implications. Mol. Asp. Med. 2012, 33, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Smith, B.L.; Christensen, E.I.; Agre, P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc. Natl. Acad. Sci. USA 1993, 90, 7275–7279. [Google Scholar] [CrossRef] [PubMed]
- Effros, R.M.; Darin, C.; Jacobs, E.R.; Rogers, R.A.; Krenz, G.; Schneeberger, E.E. Water transport and distribution of aquaporin-1 in the pulmonary airspaces. J. Appl. Physiol. 1997, 1002–1016. [Google Scholar]
- Devuyst, O.; Nielsen, S.; Cosyns, J.P.; Smith, B.L.; Agre, P.; Squifflet, J.P.; Pouthier, D.; Goffin, E. Aquaporin-1 and endothelial nitric oxide synthase expression in capillary endothelia of human peritoneum. Am. J. Physiol. 1998, 275, 234–242. [Google Scholar]
- Wen, O.; Diecke, F.P.; Iserovich, P.; Kuang, K.; Sparrow, J.; Fischbarg, J. Immunocytochemical localization of aquaporin-1 in bovine corneal endothelial cells and keratocytes. Exp. Biol. Med. 2001, 226, 463–467. [Google Scholar]
- Mobasheri, A.; Marples, D. Expression of the AQP-1 water channel in normal human tissues: A semiquantitative study using tissue microarray technology. Am. J. Physiol. Cell Physiol. 2004, 286, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Kuriyama, H.; Funahashi, T.; Shimomura, I.; Kihara, S.; Ouchi, N.; Nishida, M.; Nishizawa, H.; Matsuda, M.; Takahashi, M.; et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J. Biol. Chem. 2000, 275, 20896–20902. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Sohara, E.; Rai, T.; Ikawa, M.; Okabe, M.; Sasaki, S.; Uchida, S.; Verkman, A.S. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: Adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005, 280, 15493–15496. [Google Scholar] [CrossRef] [PubMed]
- Hibuse, T.; Maeda, N.; Funahashi, T.; Yamamoto, K.; Nagasawa, A.; Mizunoya, W.; Kishida, K.; Inoue, K.; Kuriyama, H.; Nakamura, T.; et al. Aquaporin 7 deficiency is associated with development of obesity through activation of adipose glycerol kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 10993–10998. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, T.; Yamamoto, H.; Abe, Y.; Yoshida, G.J.; Rojek, A.; Sohara, E.; Uchida, S.; Nielsen, S.; Yasui, M. Dynamic subcellular localization of aquaporin-7 in white adipocytes. FEBS Lett. 2015, 589, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Sohara, E.; Rai, T.; Miyazaki, J.; Verkman, A.S.; Sasaki, S.; Uchida, S. Defective water and glycerol transport in the proximal tubules of AQP7 knockout mice. Am. J. Physiol. Ren. Physiol. 2005, 289, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Funahashi, T.; Hibuse, T.; Nagasawa, A.; Kishida, K.; Kuriyama, H.; Nakamura, T.; Kihara, S.; Shimomura, I.; Matsuzawa, Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. USA 2004, 101, 17801–17806. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Shakibaei, M.; Marples, D. Immunohistochemical localization of aquaporin 10 in the apical membranes of the human ileum: A potential pathway for luminal water and small solute absorption. Histochem. Cell Biol. 2004, 121, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafré, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Füchtbauer, E.M.; Jelen, S.; Malmendal, A.; Fenton, R.A.; Nielsen, S. Liver-specific Aquaporin 11 knockout mice show rapid vacuolization of the rough endoplasmic reticulum in periportal hepatocytes after amino acid feeding. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N.; Li, F.K.; Lan, H.Y.; Tang, S.; Tsang, A.W.; Chan, D.T.; Leung, J.C. Expression of aquaporin-1 in human peritoneal mesothelial cells and its upregulation by glucose in vitro. J. Am. Soc. Nephrol. 2001, 12, 1036–1045. [Google Scholar] [PubMed]
- Lai, K.N.; Leung, J.C.; Chan, L.; Tang, S.; Li, F.K.; Lui, S.L.; Chan, T.M. Expression of aquaporin-3 in human peritoneal mesothelial cells and its upregulation by glucose in vitro. Kidney Int. 2002, 62, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jayaraman, S.; Wang, K.S.; Song, Y.; Yang, B.; Li, J.; Bastidas, J.A.; Verkman, A.S. Defective dietary fat processing in transgenic mice lacking aquaporin-1 water channels. Am. J. Physiol. Cell Physiol. 2001, 280, 126–134. [Google Scholar]
- Wu, H.; Chen, L.; Zhang, X.; Zhou, Q.; Li, J.M.; Berger, S.; Borok, Z.; Zhou, B.; Xiao, Z.; Yin, H.; et al. AQP5 is a new transcriptional target of Dot1a and a regulator of AQP2. PLoS ONE 2013, 8, e53342. [Google Scholar] [CrossRef] [PubMed]
- Amlal, H.; Chen, Q.; Habo, K.; Wang, Z.; Soleimani, M. Fasting downregulates renal water channel AQP2 and causes polyuria. Am. J. Physiol. Ren. Physiol. 2001, 280, 513–523. [Google Scholar]
- Skowronska, A.; Młotkowska, P.; Wojciechowicz, B.; Okrasa, S.; Nielsen, S.; Skowronski, M.T. Progesterone, estradiol, arachidonic acid, oxytocin, forskolin and cAMP influence on aquaporin 1 and 5 expression in porcine uterine explants during the mid-luteal phase of the estrous cycle and luteolysis: An in vitro study. Reprod. Biol. Endocrinol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Terris, J.; Ecelbarger, C.A.; Nielsen, S.; Knepper, M.A. Long-term regulation of four renal aquaporins in rats. Am. J. Physiol. 1996, 271, 414–422. [Google Scholar]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowronski, M.T.; Skowronska, A.; Rojek, A.; Oklinski, M.K.; Nielsen, S. Prolonged Starvation Causes Up-Regulation of AQP1 in Adipose Tissue Capillaries of AQP7 Knock-Out Mice. Int. J. Mol. Sci. 2016, 17, 1101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071101
Skowronski MT, Skowronska A, Rojek A, Oklinski MK, Nielsen S. Prolonged Starvation Causes Up-Regulation of AQP1 in Adipose Tissue Capillaries of AQP7 Knock-Out Mice. International Journal of Molecular Sciences. 2016; 17(7):1101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071101
Chicago/Turabian StyleSkowronski, Mariusz T., Agnieszka Skowronska, Aleksandra Rojek, Michal K. Oklinski, and Søren Nielsen. 2016. "Prolonged Starvation Causes Up-Regulation of AQP1 in Adipose Tissue Capillaries of AQP7 Knock-Out Mice" International Journal of Molecular Sciences 17, no. 7: 1101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071101





