“On-The-Spot” Arresting of Chondroitin Sulphate Proteoglycans: Implications for Ovarian Adenocarcinoma Recognition and Intervention
Abstract
:1. Introduction
2. Hypothesis
3. Evaluation of the Hypothesis
3.1. The Role of CS-E in Ovarian Cancer
3.2. CS-E Targeting with Antibody
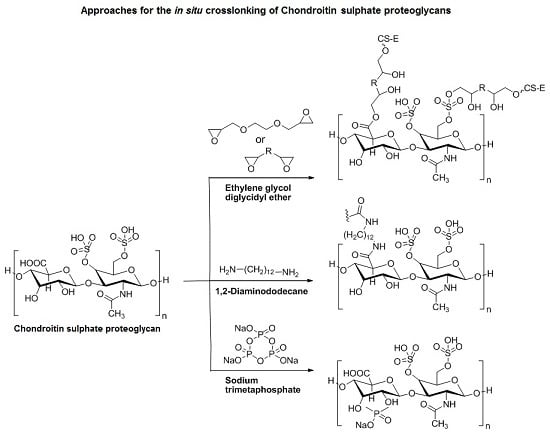
3.3. Approaches to Target and Arrest CS-E Over-Expressing Tumorous Cells
4. Conclusions and Consequences of the Hypothesis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asimakopoulou, A.P.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K. The biological role of chondroitin sulfate in cancer and chondroitin-based anticancer agents. In Vivo 2008, 22, 385–389. [Google Scholar] [PubMed]
- Handley, C.J.; Samiric, T.; Ilic, M.Z. Structure, metabolism; and tissue roles of chondroitin sulfate proteoglycans. Adv. Pharmacol. 2006, 53, 219–232. [Google Scholar] [PubMed]
- Afratis, N.; Gialeli, C.; Nikitovic, D.; Tsegenidis, T.; Karousou, E.; Theocharis, A.D.; Pavão, M.S.; Tzanakakis, G.N.; Karamanos, N.K. Glycosaminoglycans: Key players in cancer cell biology and treatment. FEBS J. 2012, 279, 1177–1197. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Watson, K.; Ingber, D.; Hanahan, D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature 1989, 339, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Tsolakis, I.; Tzanakakis, G.N.; Karamanos, N.K. Chondroitin sulfate as a key molecule in the development of atherosclerosis and cancer progression. Adv. Pharmacol. 2006, 53, 281–295. [Google Scholar] [PubMed]
- Wegrowski, Y.; Maquart, F.X. Chondroitin sulfate proteoglycans in tumor progression. Adv. Pharmacol. 2006, 53, 297–321. [Google Scholar] [PubMed]
- Vallen, M.J.E.; van der Steen, S.C.H.A.; van Tilborg, A.A.G.; Massugar, L.F.A.G.; van Kuppervelt, T.H. Sulfated sugars in the extracellular matrix orchestrate ovarian cancer development: ‘When sweet turns sour’. Gynecol. Oncol. 2014, 135, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Deepa, S.S.; Umehara, Y.; Higashiyama, S.; Itoh, N.; Sugahara, K. Specific Molecular Interactions of Oversulfated Chondroitin Sulfate E with Various Heparin-binding Growth Factors. J. Biol. Chem. 2002, 277, 43707–43716. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, S.; Sugahara, K. Glycosaminoglycans are functional ligands for receptor for advanced glycation end-products in tumors. FEBS J. 2013, 280, 2462–2470. [Google Scholar] [CrossRef] [PubMed]
- Pantazaka, E.; Papadimitriou, E. Chondriotin sulphate-cell membrane effectors as regulators of growth factor-mediated vascular and cancer cell migration. Biochem. Biophis. Acta 2014, 1840, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Ten Dam, G.B.; van de Westerlo, E.M.A.; Purushothaman, A.; Stan, R.V.; Bulten, J.; Sweep, F.C.G.J.; Massuger, L.F.; Sugahara, K.; van Kuppevelt, T.H. Antibody GD3G7 Selected against Embryonic Glycosaminoglycans Defines Chondroitin Sulfate-E Domains Highly Up-Regulated in Ovarian Cancer and Involved in Vascular Endothelial Growth Factor Binding. Am. J. Pathol. 2007, 171, 1324–1333. [Google Scholar] [PubMed]
- Vallen, M.J.E.; Schmidt, S.; Oosterhof, A.; Bulten, J.; Massuger, L.F.A.G.; van Kuppevelt, T.H. Primary Ovarian Carcinomas and Abdominal Metastasis Contain 4,6-Disulfated Chondroitin Sulfate Rich Regions, Which Provide Adhesive Properties to Tumor Cells. PLoS ONE 2014, 9, e111806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwanicki, M.P.; Davidowitz, R.A.; Ng, M.R.; Besser, A.; Muranen, T.; Merritt, M.; Danuser, G.; Ince, T.; Brugge, J.S. Ovarian Cancer Spheroids Use Myosin-Generated Force to Clear the Mesothelium. Cancer Discov. 2011, 12, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H. Chitosan Based Polyelectrolyte Complexes as Potential Carrier Materials in Drug Delivery Systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, O.A.; da Silva, C.C.; Pineda, E.A.G.; Hechenleitner, A.A.W. Synthesis and Characterization of Phosphated Crosslinked Chondroitin Sulfate: Potential Ingredient for Specific Drug Delivery. Acta Farm. Bonaer. 2005, 24, 234–238. [Google Scholar]
- Jensen, M.; Birch Hansen, P.; Murdan, S.; Frokjaer, S.; Florence, A.T. Loading into and electro-stimulated release of peptides and proteins from chondroitin 4-sulphate hydrogels. Eur. J. Pharm. Sci. 2002, 15, 139–148. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, B.H.; Wang, L.F.; Chen, J.S. Characterization of chondroitin sulfate and its interpenetrating polymer network hydrogels for sustained-drug release. Int. J. Pharm. 2007, 329, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.; Di-Capua, N.; Rubinstein, A. Cross-linked chondroitin sulphate: Characterization for drug delivery purposes. Biomaterials 1995, 16, 473–478. [Google Scholar] [CrossRef]
- Sample Availability: This is a conceptual study and hence physical samples of the compounds are not available from the authors.







| CS Type | Disaccharide Unit | Substituents |
|---|---|---|
| CS-A | A = [β1-4]-d-glucuronic acid B = [β1-3]-N-acetyl-d-galactosamine | R2 = H R4 =  R6 = H |
| CS-B/DS | A = [β1-4]-l-iduronic acid B = [α1-3]-N-acetyl-d-galactosamine | R2 = H R4 =  R6 = H |
| CS-C | A = [β1-4]-d-glucuronic acid B = [β1-3]-N-acetyl-d-galactosamine | R2 = H R4 = H R6 =  |
| CS-D | A = [β1-4]-d-glucuronic acid B = [β1-3]-N-acetyl-d-galactosamine | R2 =  R4 = H R6 =  |
| CS-E | A = [β1-4]-d-glucuronic acid B = [β1-3]-N-acetyl-d-galactosamine | R2 = H R4 =  R6 =  |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradeep, P.; Choonara, Y.E.; Kumar, P.; Pillay, V. “On-The-Spot” Arresting of Chondroitin Sulphate Proteoglycans: Implications for Ovarian Adenocarcinoma Recognition and Intervention. Int. J. Mol. Sci. 2016, 17, 1136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071136
Pradeep P, Choonara YE, Kumar P, Pillay V. “On-The-Spot” Arresting of Chondroitin Sulphate Proteoglycans: Implications for Ovarian Adenocarcinoma Recognition and Intervention. International Journal of Molecular Sciences. 2016; 17(7):1136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071136
Chicago/Turabian StylePradeep, Priyamvada, Yahya E. Choonara, Pradeep Kumar, and Viness Pillay. 2016. "“On-The-Spot” Arresting of Chondroitin Sulphate Proteoglycans: Implications for Ovarian Adenocarcinoma Recognition and Intervention" International Journal of Molecular Sciences 17, no. 7: 1136. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17071136