Transfusion of Red Blood Cells to Patients with Sepsis
Abstract
:1. Introduction
2. Sepsis
3. Rationale for Packed Red Blood Cell (pRBC) Transfusion during Sepsis
4. Have Transfused RBCs Increased Oxygen Delivery?
5. Does the Tissue Need More Oxygen during Sepsis?
6. Complications of pRBC Transfusion during Sepsis
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 2,3-DPG | 2,3-Diphosphoglycerate |
| ATP | Adenosine triphosphate |
| C(a–v)O2 | Arteriovenous oxygen difference |
| DO2 | Oxygen delivery |
| EGDT | Early goal-directed therapy |
| Hb | Hemoglobin |
| HbO2 | Oxyhemoglobin |
| PaO2 | Arterial oxygen partial pressure |
| pRBCs | Packed red blood cells |
| PvO2 | Mixed venous oxygen tension |
| SaO2 | Arterial oxygen saturation |
| SvO2 | Mixed venous oxygen saturation |
| TLR | Toll-like receptors |
| TNF | Tumor necrosis factor |
| VO2 | Oxygen consumption |
References
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Sepsis Fact Sheet, National Institute of General Medical Sciences. Available online: https://www.nigms.nih.gov/education/pages/factsheet_sepsis.aspx (accessed on 15 May 2017).
- Parrillo, J.E.; Parker, M.M.; Natanson, C.; Suffredini, A.F.; Danner, R.L.; Cunnion, R.E.; Ognibene, F.P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 1990, 113, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Carlet, J.M.; Masur, H.; Gerlach, H.; Calandra, T.; Cohen, J.; Gea-Banacloche, J.; Keh, D.; Marshall, J.C.; Parker, M.M.; et al. Surviving sepsis campaign guidelines for management of severe sepsis and septic shock. Crit. Care Med. 2004, 32, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Milsark, I.W.; Cerami, A.C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science 1985, 229, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Sibbald, W.J.; Sprung, C.L. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 1992, 101, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Thiemermann, C. Nitric oxide and septic shock. Gen. Pharmacol. 1997, 29, 159–166. [Google Scholar] [CrossRef]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; de Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Lomas, J.L.; Grutkoski, P.S.; Chung, C.S. Pathological aspects of apoptosis in severe sepsis and shock? Int. J. Biochem. Cell Biol. 2002, 35, 7–15. [Google Scholar] [CrossRef]
- Beltrán, B.; Orsi, A.; Clementi, E.; Moncada, S. Oxidative stress and S-nitrosylation of proteins in cells. Br. J. Pharmacol. 2000, 129, 953–960. [Google Scholar]
- Sprung, C.L.; Caralis, P.V.; Marcial, E.H.; Pierce, M.; Gelbard, M.A.; Long, W.M.; Duncan, R.C.; Tendler, M.D.; Karpf, M. The effects of high-dose corticosteroids in patients with septic shock. N. Engl. J. Med. 1984, 311, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Anzueto, A.; Gutierrez, G.; Tessler, S.; San Pedro, G.; Wunderink, R.; Dal Nogare, A.; Nasraway, S.; Berman, S.; Cooney, R.; et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet 1998, 351, 929–933. [Google Scholar] [CrossRef]
- Bernard, G.R.; Vincent, J.L.; Laterre, P.F.; LaRosa, S.; Dhainaut, J.F.; Lopez-Rodriguez, A.; Steingrub, J.; Garber, G.; Helterbrand, J.; Ely, E.W.; et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Sébille, V.; Charpentier, C.; Bollaert, P.E.; François, B.; Korach, J.M.; Capellier, G.; Cohen, Y.; Azoulay, E.; Troché, G.; et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002, 288, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive insulin therapy in the critically ill patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Rivers, E.P.; Nguyen, H.B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.D.; Tomlanovich, M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Surviving Sepsis Campaign. Available online: http://www.survivingsepsis.org (accessed on 29 May 2017).
- Barcelona Declaration—Surviving Sepsis Campaign. Available online: http://www.survivingsepsis.org/SiteCollectionDocuments/About-Barcelona-Declaration.pdf (accessed on 9 July 2017).
- Ranieri, V.M.; Thompson, B.T.; Barie, P.S.; Dhainaut, J.F.; Douglas, I.S.; Finfer, S.; Gårdlund, B.; Marshall, J.C.; Rhodes, A.; Artigas, A.; et al. Drotrecogin alfa (activated) in adults with septic shock. N. Engl. J. Med. 2012, 366, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Sprung, C.L.; Annane, D.; Keh, D.; Moreno, R.P.; Singer, M.; Freivogel, K.; Weiss, Y.G.; Benbenishty, J.; Kalenka, A.; Forst, H.; et al. Hydrocortisone therapy for patients with septic shock. N. Engl. J. Med. 2008, 358, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Finfer, S.; Chittock, D.R.; Su, S.Y.S.; Blair, D.; Foster, D.; Dhingra, V.; Bellomo, R.; Cook, D.; Dodek, P.; Henderson, W.R.; et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009, 360, 1283–1297. [Google Scholar] [PubMed]
- Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; LoVecchio, F.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [PubMed]
- Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Cameron, P.A.; Cooper, D.J.; Higgins, A.M.; Holdgate, A.; Howe, B.D.; Webb, S.A.R.; et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014, 371, 1496–1506. [Google Scholar] [PubMed]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of early, goal-directed resuscitation for septic shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; Ngerng, W.; See, K.; Tay, C.; Kiong, T.; Lim, H.; Chew, M.; Yip, H.; Tan, A.; Khalizah, H.; et al. Characteristics and outcomes of culture-negative versus culture-positive severe sepsis. Crit. Care 2013, 17, R202. [Google Scholar] [CrossRef] [PubMed]
- Dhainaut, J.F. Role of clinical evaluation committees in sepsis trials: From “valid cohort” assessment to subgroup analysis. Crit. Care 2009, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M. Sepsis definitions task force developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Kandel, G.; Aberman, A. Mixed venous oxygen saturation. Arch. Intern. Med. 1983, 143, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.P. M.; van Lieshout, J.J.; Jenstrup, M.; Pott, F.; Secher, N.H. Postural effects on cardiac output and mixed venous oxygen saturation in humans. Exp. Physiol. 2003, 88, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Marino, P.L. Oxygen and Carbon Dioxide Transport. In The ICU Book, 2nd ed.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 1998; p. 28. [Google Scholar]
- Hayes, M.A.; Timmins, A.C.; Yau, E.H.; Palazzo, M.; Watson, D.; Hinds, C.J. Oxygen transport patterns in patients with sepsis syndrome or septic shock: Influence of treatment and relationship to outcome. Crit. Care Med. 1997, 25, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, W.C.; Appel, P.L.; Kram, H.B. Role of oxygen debt in the development of organ failure sepsis, and death in high-risk surgical patients. Chest 1992, 102, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Levy, M.M.; Smith, P.; Takiguchi, S.A.; Miyasaki, A.; Myers, S.A. Effect of maximizing oxygen delivery on morbidity and mortality rates in critically ill patients: A prospective, randomized, controlled study. Crit. Care Med. 1993, 21, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.A.; Timmins, A.C.; Yau, E.H.; Palazzo, M.; Hinds, C.J.; Watson, D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N. Engl. J. Med. 1994, 330, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Brazzi, L.; Pelosi, P.; Latini, R.; Tognoni, G.; Pesenti, A.; Fumagalli, R. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N. Engl. J. Med. 1995, 333, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Chun, B.C.; Kwon, S.S.; Yoon, Y.K.; Choi, W.S.; Sohn, J.W.; Peck, K.R.; Kim, Y.S.; Choi, Y.H.; Choi, J.Y.; et al. Red blood cell transfusions are associated with lower mortality in patients with severe sepsis and septic shock. Crit. Care Med. 2012, 40, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, R.B.; Viele, M.K.; Feiner, J.; Kelley, S.; Lieberman, J.; Noorani, M.; Leung, J.M.; Fisher, D.M.; Murray, W.R.; Toy, P.; et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998, 279, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, G.; Pohil, R.J. Oxygen consumption is linearly related to O2 supply in critically ill patients. J. Crit. Care 1986, 1, 45–53. [Google Scholar] [CrossRef]
- Hanique, G.; Dugernier, T.; Laterre, P.F.; Dougnac, A.; Roeseler, J.; Reynaert, M.S. Significance of pathologic oxygen supply dependency in critically ill patients: Comparison between measured and calculated methods. Intensive Care Med. 1994, 20, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.P.; Fabre, J.E.; Baigorri, F.; Coste, J.; Annat, G.; Artigas, A.; Nitenberg, G.; Dhainaut, J.F. Lack of oxygen supply dependency in patients with severe sepsis: A study of oxygen delivery increased by military antishock trouser and dobutamine. Chest 1994, 106, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P. Whole Body and Organ Measures of O2 Availability. In Clinical Trials for the Treatment of Sepsis; Update in Intensive Care and Emergency Medicine; Springer: Berlin/Heidelberg, Germany, 1995; Volume 19, pp. 106–121. [Google Scholar]
- Marik, P.E.; Varon, J. Early goal-directed therapy: On terminal life support? Am. J. Emerg. Med. 2010, 28, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Ince, C.; Sinaasappel, M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit. Care Med. 1999, 27, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Bennett-Guerrero, E.; Veldman, T.H.; Doctor, A.; Telen, M.J.; Ortel, T.L.; Reid, T.S.; Mulherin, M.A.; Zhu, H.; Buck, R.D.; Califf, R.M.; et al. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA 2007, 104, 17063–17068. [Google Scholar] [CrossRef] [PubMed]
- Goyette, R.E.; Key, N.S.; Ely, E.W. Hematologic changes in sepsis and their therapeutic implications. Semin. Respir. Crit. Care Med. 2004, 25, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Stan, A.; Zsigmond, E. The restoration in vivo of 2,3-diphosphoglycerate (2,3-DPG) in stored red cells, after transfusion. The levels of red cells 2,3-DPG. Rom. J. Intern. Med. 2009, 47, 173–177. [Google Scholar] [PubMed]
- Kim, J.; Lee, H.; Shin, S. Advances in the measurement of red blood cell deformability: A brief review. J. Cell Biotechnol. 2015, 1, 63–79. [Google Scholar] [CrossRef]
- Moutzouri, A.G.; Skoutelis, A.T.; Gogos, C.A.; Missirlis, Y.F.; Athanassiou, G.M. Red blood cell deformability in patients with sepsis: A marker for prognosis and monitoring of severity. Clin. Hemorheol. Microcirc. 2007, 36, 291–299. [Google Scholar] [PubMed]
- Hurd, T.C. Red blood cell deformability in human and experimental sepsis. Arch. Surg. 1988, 123, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Serroukh, Y.; Djebara, S.; Lelubre, C.; Zouaoui Boudjeltia, K.; Biston, P.; Piagnerelli, M. Alterations of the erythrocyte membrane during sepsis. Crit. Care Res. Pract. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Blasi, B.; D’Alessandro, A.; Ramundo, N.; Zolla, L. Red blood cell storage and cell morphology. Transfus. Med. 2012, 22, 90–96. [Google Scholar] [CrossRef] [PubMed]
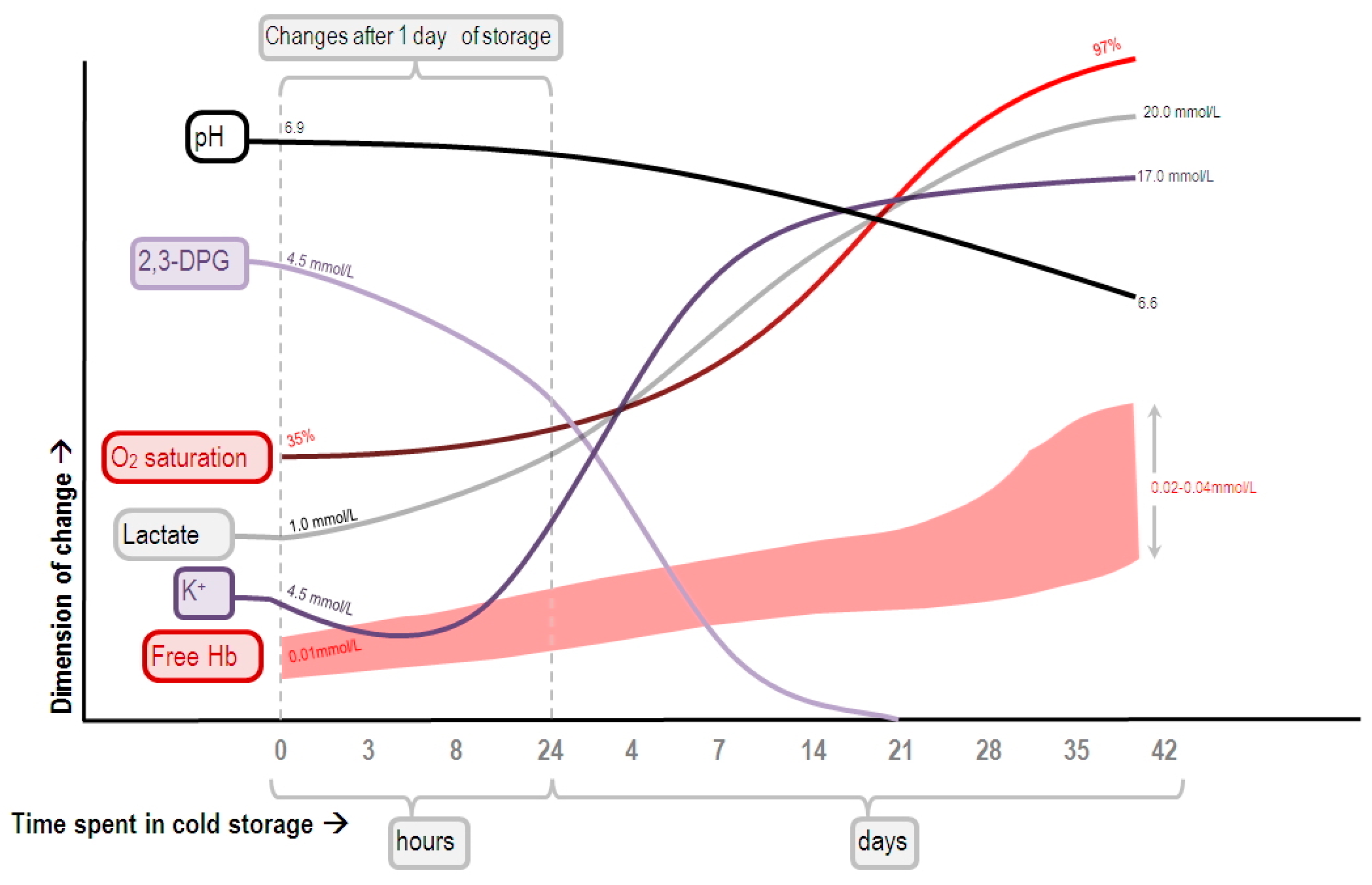
- Kor, D.J.; van Buskirk, C.M.; Gajic, O. Red blood cell storage lesion. Bosn. J. Basic. Med. Sci. 2009, 9, 21–27. [Google Scholar] [PubMed]
- Riccio, D.A.; Zhu, H.; Foster, M.W.; Huang, B.; Hofmann, C.L.; Palmer, G.M.; McMahon, T.J. Renitrosylation of banked human red blood cells improves deformability and reduces adhesivity. Transfusion 2015, 55, 2452–2463. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Kriebardis, A.G.; Rinalducci, S.; Antonelou, M.H.; Hansen, K.C.; Papassideri, I.S.; Zolla, L. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2014, 55, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Raat, N.J.H.; Ince, C. Oxygenating the microcirculation: The perspective from blood transfusion and blood storage. Vox Sang. 2007, 93, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E. Effect of stored-blood transfusion on oxygen delivery in patients with sepsis. JAMA 1993, 269, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.S.; McArdle, F.; McLellan, S.A.; Maciver, C.; Maginnis, M.; Prescott, R.J.; McClelland, D.B. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit. Care Med. 2004, 32, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Liu, S.H.; Chao, C.H.; Chan, Y.L.; Tsai, T.C.; Chen, L.M.; Wu, C.C.; Chen, K.F. STROBE-compliant article: Blood transfusion within the first 24 h of hospitalization did not impact mortality among patients with severe sepsis. Medicine 2016, 95, e2601–e2607. [Google Scholar] [CrossRef] [PubMed]
- Sadaka, F.; Aggu-Sher, R.; Krause, K.; O’Brien, J.; Armbrecht, E.S.; Taylor, R.W. The effect of red blood cell transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann. Intensive Care 2011, 1, 46. [Google Scholar] [CrossRef] [PubMed]
- Kopterides, P.; Theodorakopoulou, M.; Nikitas, N.; Ilias, I.; Vassiliadi, D.A.; Orfanos, S.E.; Tsangaris, I.; Maniatis, N.A.; Tsantes, A.E.; Travlou, A.; et al. Red blood cell transfusion affects microdialysis-assessed interstitial lactate/pyruvate ratio in critically ill patients with late sepsis. Intensive Care Med. 2012, 38, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, J.; Hébert, P.C.; Fergusson, D.A.; Tinmouth, A.; Cook, D.J.; Marshall, J.C.; Clayton, L.; McIntyre, L.; Callum, J.; Turgeon, A.F.; et al. Age of transfused blood in critically ill adults. N. Engl. J. Med. 2015, 372, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Donati, A.; Damiani, E.; Luchetti, M.; Domizi, R.; Scorcella, C.; Carsetti, A.; Gabbanelli, V.; Carletti, P.; Bencivenga, R.; Vink, H.; et al. Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in patients with sepsis: A pilot study. Crit. Care 2014, 18, R33. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.G.; Hofmann, A.; Cabrales, P.; Intaglietta, M. Perfusion vs. oxygen delivery in transfusion with “fresh” and ‘old’ red blood cells: The experimental evidence. Transfus. Apher. Sci. 2010, 43, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, P.; Tsai, A.G.; Intaglietta, M. Hemorrhagic shock resuscitation with carbon monoxide saturated blood. Resuscitation 2007, 72, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Salazar Vázquez, B.Y.; Cabrales, P.; Tsai, A.G.; Johnson, P.C.; Intaglietta, M. Lowering of blood pressure by increasing hematocrit with non–nitric oxide–scavenging red blood cells. Am. J. Respir. Cell Mol. Biol. 2008, 38, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, R.; Tsai, A.G.; Salazar Vázquez, B.Y.; Cabrales, P.; Hofmann, A.; Meier, J.; Shander, A.; Spahn, D.R.; Friedman, J.M.; Tartakovsky, D.M.; et al. Posttransfusion increase of hematocrit per se does not improve circulatory oxygen delivery due to increased blood viscosity. Anesth. Analg. 2017, 124, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.G.; Vázquez, B.Y. S.; Hofmann, A.; Acharya, S.A.; Intaglietta, M. Supra-plasma expanders. J. Infus. Nurs. 2015, 38, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Holst, L.B.; Haase, N.; Wetterslev, J.; Wernerman, J.; Guttormsen, A.B.; Karlsson, S.; Johansson, P.I.; Åneman, A.; Vang, M.L.; Winding, R.; et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N. Engl. J. Med. 2014, 371, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, C.; Sonneville, R.; Adrie, C.; Gros, A.; Darmon, M.; Bouadma, L.; Timsit, J.F. Impact of transfusion on patients with sepsis admitted in intensive care unit: A systematic review and meta-analysis. Ann. Intensive Care 2016, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brealey, D.; Brand, M.; Hargreaves, I.; Heales, S.; Land, J.; Smolenski, R.; Davies, N.A.; Cooper, C.E.; Singer, M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002, 360, 219–223. [Google Scholar] [CrossRef]
- Singer, M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Textoris, J.; Fouché, L.; Wiramus, S.; Antonini, F.; Tho, S.; Martin, C.; Leone, M. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit. Care 2011, 15, R176. [Google Scholar] [CrossRef] [PubMed]
- Dyson, A.; Stidwill, R.P.; Taylor, V.; Singer, M. Tissue oxygen monitoring in rodent models of shock. Am. J. Physiol. 2007, 293, H526–H533. [Google Scholar] [CrossRef] [PubMed]
- Arulkumaran, N.; Deutschman, C.S.; Pinsky, M.R.; Zuckerbraun, B.; Schumacker, P.T.; Gomez, H.; Gomez, A.; Murray, P.; Kellum, J.A.; ADQI XIV Workgroup. Mitochondrial function in sepsis. Shock 2016, 45, 271–281. [Google Scholar] [CrossRef] [PubMed]
- James, J.H.; Fang, C.H.; Schrantz, S.J.; Hasselgren, P.O.; Paul, R.J.; Fischer, J.E. Linkage of aerobic glycolysis to sodium-potassium transport in rat skeletal muscle. Implications for increased muscle lactate production in sepsis. J. Clin. Investig. 1996, 98, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.; Gibot, S.; Franck, P.; Cravoisy, A.; Bollaert, P.-E. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: A prospective study. Lancet 2005, 365, 871–875. [Google Scholar] [CrossRef]
- Michaeli, B.; Martinez, A.; Revelly, J.P.; Cayeux, M.C.; Chioléro, R.L.; Tappy, L.; Berger, M.M. Effects of endotoxin on lactate metabolism in humans. Crit. Care 2012, 16, R139. [Google Scholar] [CrossRef] [PubMed]
- Sadaka, F. Red Blood Cell Transfusion in Sepsis: A Review. J. Blood Disord. Transfus. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Toy, P.; Gajic, O.; Bacchetti, P.; Looney, M.R.; Gropper, M.A.; Hubmayr, R.; Lowell, C.A.; Norris, P.J.; Murphy, E.L.; Weiskopf, R.B.; et al. Transfusion-related acute lung injury: Incidence and risk factors. Blood 2012, 119, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Johnson, D.J.; Scott, A.V.; Tobian, A.A.R.; Ness, P.M.; Nagababu, E.; Frank, S.M. Red blood cells stored 35 days or more are associated with adverse outcomes in high-risk patients. Transfusion 2016, 56, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Hod, E.A.; Brittenham, G.M.; Billote, G.B.; Francis, R.O.; Ginzburg, Y.Z.; Hendrickson, J.E.; Jhang, J.; Schwartz, J.; Sharma, S.; Sheth, S.; et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood 2011, 118, 6675–6682. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H.; Bradbury, R.S.; Fulford, A.J.; Jallow, A.T.; Wegmüller, R.; Prentice, A.M.; Cerami, C. Oral iron acutely elevates bacterial growth in human serum. Sci. Rep. 2015, 5, 16670. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Crit. Care Med. 2017, 45, 486–552. [Google Scholar] [CrossRef] [PubMed]
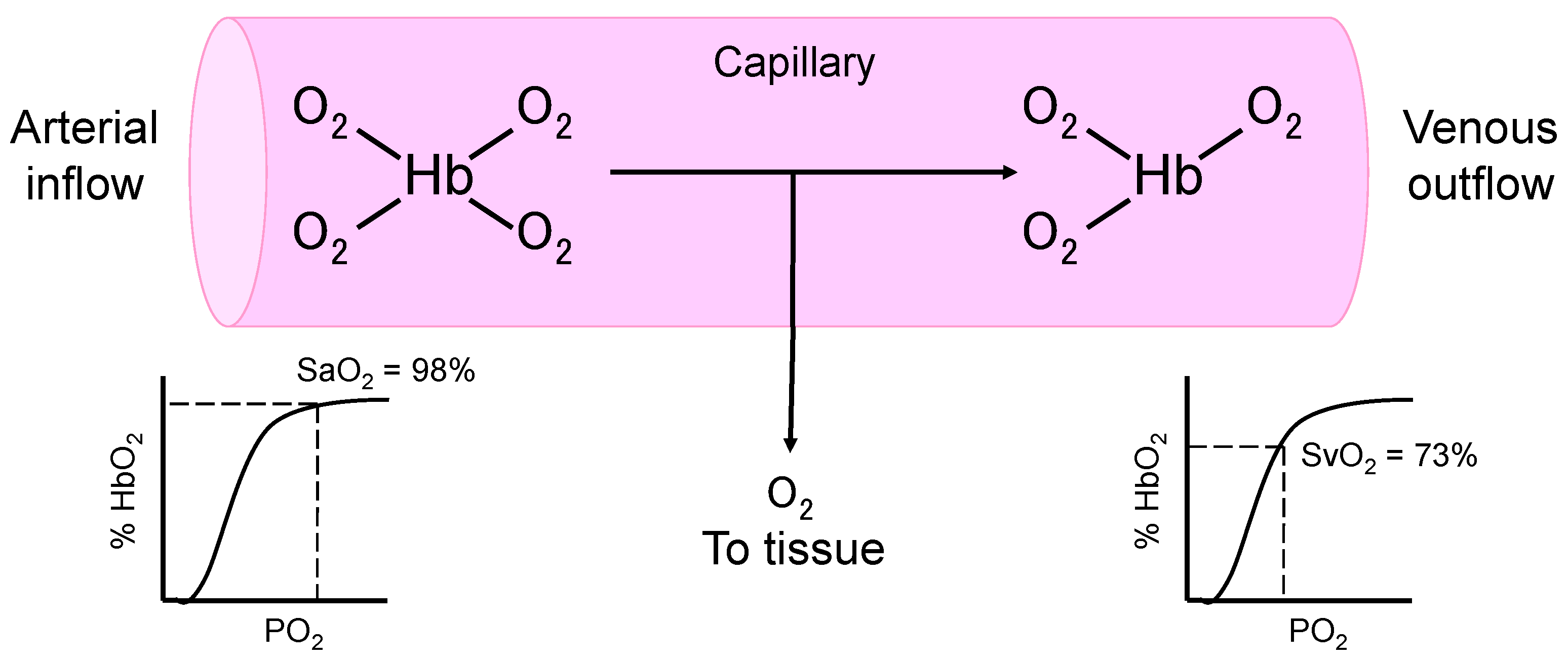
| Cardiac index (L/min/m2) = Cardiac output/body surface area |
| Arterial oxygen content (mL/dL) = 1.39 × Hb (g/dL) × SaO2 + 0.0225 × PaO2 (kPa) |
| Mixed venous oxygen content (mL/dL) = 1.39 × Hb (g/dL) × SvO2 + 00225 × PvO2 (kPa) |
| C(a–v)O2 = arterial oxygen content − mixed venous oxygen content |
| DO2 (mL/min/m2) = cardiac index × arterial oxygen content × 10 |
| VO2 (mL/min/m2) = cardiac index × C(a–v)O2 × 10 |
| Oxygen extraction ratio = C(a–v)O2/arterial oxygen content |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, Y.-L.; Han, S.-T.; Li, C.-H.; Wu, C.-C.; Chen, K.-F. Transfusion of Red Blood Cells to Patients with Sepsis. Int. J. Mol. Sci. 2017, 18, 1946. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091946
Chan Y-L, Han S-T, Li C-H, Wu C-C, Chen K-F. Transfusion of Red Blood Cells to Patients with Sepsis. International Journal of Molecular Sciences. 2017; 18(9):1946. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091946
Chicago/Turabian StyleChan, Yi-Ling, Shih-Tsung Han, Chih-Huang Li, Chin-Chieh Wu, and Kuan-Fu Chen. 2017. "Transfusion of Red Blood Cells to Patients with Sepsis" International Journal of Molecular Sciences 18, no. 9: 1946. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18091946





