The Influences of Soybean Agglutinin and Functional Oligosaccharides on the Intestinal Tract of Monogastric Animals
Abstract
:1. Introduction
2. Soybean Agglutinin
2.1. Soybean Agglutinin (SBA) Structure
2.2. SBA Anti-Nutritional Functions
2.2.1. SBA and Intestinal Morphological and Structural Patterns
2.2.2. SBA and Intestinal Mechanical Barrier Function
2.2.3. SBA and the Intestinal Mucosal Immune System
2.2.4. SBA and the Balance of Intestinal Flora
2.3. The Positive Effects of SBA
3. Functional Oligosaccharides
3.1. Galacto-Oligosaccharides
3.2. Fructo-Oligosaccharide
3.3. Mannan-Oligosaccharide
3.4. Chitosan Oligosaccharides
3.5. Cello-Oligosaccharide
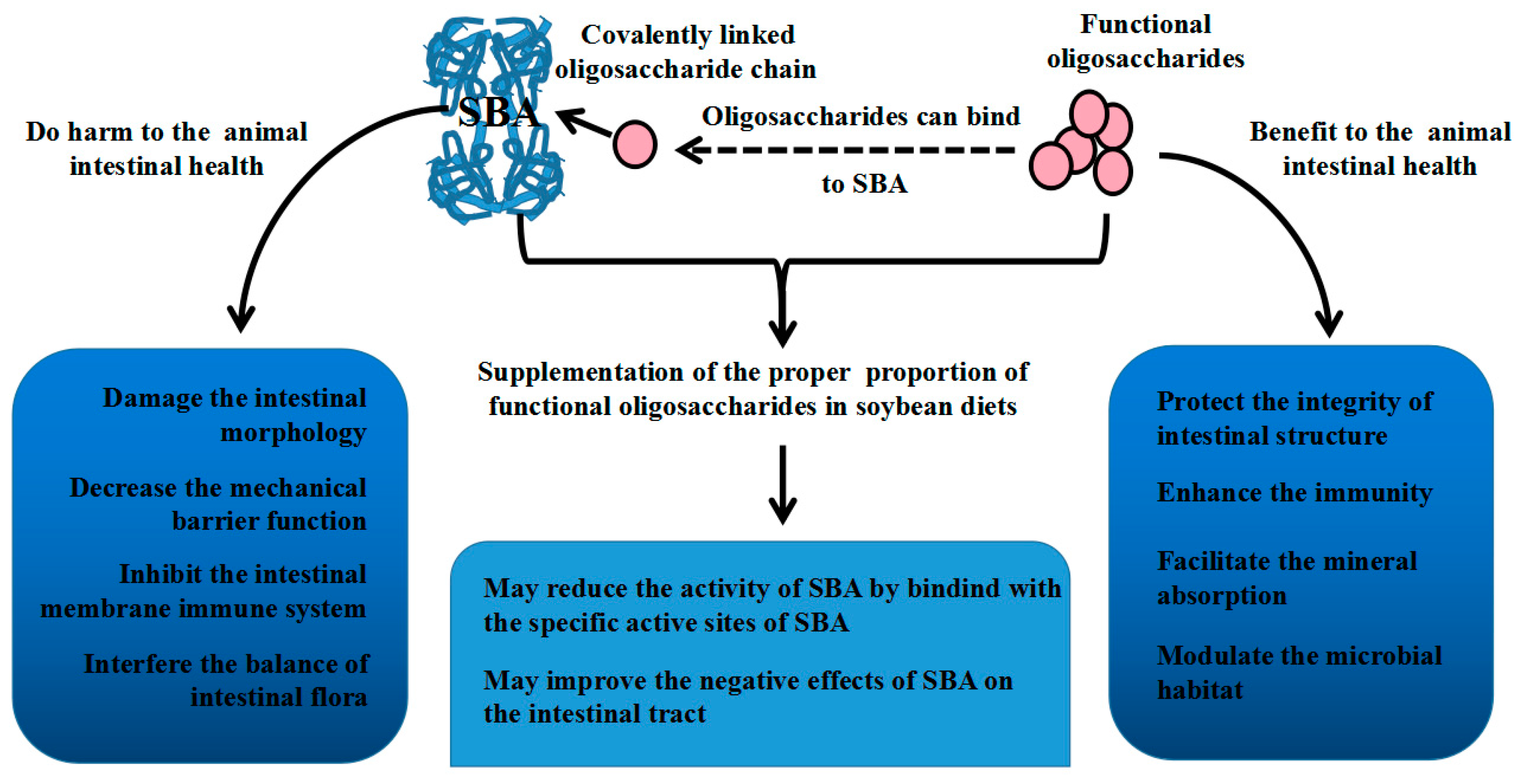
4. Relationship between SBA and Oligosaccharides
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chatterjee, M.; Mandal, D.K. Kinetic analysis of subunit oligomerization of the legume lectin soybean agglutinin. Biochemistry 2003, 42, 12217–12222. [Google Scholar] [CrossRef] [PubMed]
- Fasina, Y.O.; Garlich, J.D.; Classen, H.L.; Ferket, P.R.; Havenstein, G.B.; Grimes, J.L.; Qureshi, M.A.; Christensent, V.L. Response of turkey poults to soybean lectin levels typically encountered in commercial diets. 1. Effect on growth and nutrient digestibility. Poult. Sci. 2004, 83, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Qin, G.; Zhao, Y.; Wang, J.; Liu, F.; Che, D. Effects of soybean agglutinin on mechanical barrier function and tight junction protein expression in intestinal epithelial cells from piglets. Int. J. Mol. Sci. 2013, 14, 21689–21704. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhao, Y.; Yuan, Z.; Farouk, M.H.; Zhang, S.; Bao, N.; Qin, G. The integrins involved in soybean agglutinin-induced cell cycle alterations in IPEC-J2. Mol. Cells 2017, 40, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.; Pusztai, A. Toxicity of kidney bean (Phaseolus vulgaris) in rats: Changes in intestinal permeability. Digestion 1985, 32, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, A.; Grant, G.; Spencer, R.J.; Duguid, T.J.; Brown, D.S.; Ewen, S.W.B.; Peumans, W.J.; van Damme, E.J.M.; Bardocz, S. Kidney bean lectin-induced Escherichia coli overgrowth in the small intestine is blocked by GNA, a mannose-specific lectin. J. Appl. Bacteriol. 1993, 75, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.; Ewen, S.; Bardocz, S.; Brown, D.; Dorward, P.; Watt, W.; Stewart, J.; Pusztai, A. Local (gut) and systemic responses of rats to dietary soybean (Glycine max) proteins. In Proceedings of the Recent Advances of Research in Antinutritional Factors in Legume Seeds, Wageningen, The Netherlands, 23–25 November 1989; Huisman, J., van der Poel, T., Liener, I., Eds.; Pudoc: Wageningen, The Netherlands, 1989; pp. 34–38. [Google Scholar]
- Zhao, C.; Wu, Y.; Liu, X.; Liu, B.; Cao, H.; Yu, H.; Sarker, S.D.; Nahar, L.; Xiao, J. Functional properties, structural studies and chemo-enzymatic synthesis of oligosaccharides. Trends Food Sci. Technol. 2017, 66, 135–145. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Patel, S.; Goyal, A. Functional oligosaccharides: Production, properties and applications. World J. Microbiol. Biotechnol. 2011, 27, 1119–1128. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Jung, J.H.; Kim, I.H. Effect of mannan oligosaccharides and fructan on growth performance, nutrient digestibility, blood profile, and diarrhea score in weanling pigs1. J. Anim. Sci. 2012, 90, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Stillmark, H. Über Ricin, ein Giftiges Ferment aus den Samen von Ricinus comm. L. und Einigen Anderen Euphorbiaceen. Ph.D. Thesis, University of Tartu, Tartu, Estonia, 1888. [Google Scholar]
- Liener, I.E.; Paelansch, M.J. Purification of a toxic substance from defatted soy bean flonr. J. Biol. Chem. 1952, 197, 29–36. [Google Scholar] [PubMed]
- Sinha, S.; Surolia, A. Attributes of glycosylation in the establishment of the unfolding pathway of soybean agglutinin. Biophys. J. 2007, 92, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Dessen, A.; Gupta, D.; Sabesan, S.; Brewer, C.F.; Sacchettini, J.C. X-ray crystal structure of the soybean agglutinin cross-linked with a biantennary analog of the blood group I carbohydrate antigen. Biochemistry 1995, 34, 4933–4942. [Google Scholar] [CrossRef] [PubMed]
- Lotan, R.; Siegelman, H.W.; Lis, H.; Sharon, N. Subunit structure of soybean agglutinin. J. Biol. Chem. 1974, 249, 1219–1224. [Google Scholar] [PubMed]
- Alam, P.; Naseem, F.; Abdelhameed, A.S.; Khan, R.H. Effect of galactose on acid induced molten globule state of soybean agglutinin: Biophysical approach. J. Mol. Struct. 2015, 1099, 149–153. [Google Scholar] [CrossRef]
- Rao, V.S.R.; Lam, K.; Qasba, P.K. Three dimensional structure of the soybean agglutinin-Gal/GalNAc complexes by homology modeling. J. Biomol. Struct. Dyn. 1998, 15, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, D.; Qiao, S. Effects of soybean agglutinin on nitrogen metabolism and on characteristics of intestinal tissues and pancreas in rats. Arch. Anim. Nutr. 2003, 57, 369–380. [Google Scholar] [CrossRef]
- Pusztai, A.; Grant, G.; Bardocz, S.; Gelencser, E.; Hajos, G.Y. Novel dietary strategy for overcoming the antinutritional effects of soyabean whey of high agglutinin content. Br. J. Nutr. 2007, 77, 933–945. [Google Scholar] [CrossRef]
- Draaijer, M.; Koninkx, J.; Hendriks, H.; Kik, M.; Van Dijk, J.; Mouwen, J. Actin cytoskeletal lesions in differentiated human colon carcinoma Caco-2 cells after exposure to soybean agglutinin. Biol. Cell 1989, 65, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.G.C.J.M.; Van den Ingh, T.S.G.A.M.; Krogdahl, Å.; Olli, J.; Koninkx, J.F.J.G. Binding of soybean agglutinin to small intestinal brush border membranes and brush border membrane enzyme activities in Atlantic salmon (Salmo salar). Aquaculture 1990, 91, 163–170. [Google Scholar] [CrossRef]
- Van den Ingh, T.S.G.A.M.; Krogdahl, Å.; Olli, J.J.; Hendriks, H.G.C.J.M.; Koninkx, J.G.J.F. Effects of soybean-containing diets on the proximal and distal intestine in Atlantic salmon (Salmo salar): A morphological study. Aquaculture 1991, 94, 297–305. [Google Scholar] [CrossRef]
- Maenz, D.D.; Irish, G.G.; Classen, H.L. Carbohydrate-binding and agglutinating lectins in raw and processed soybean meals. Anim. Feed Sci. Technol. 1999, 76, 335–343. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Classen, H.L.; Garlich, J.D.; Black, B.L.; Ferket, P.R.; Uni, Z.; Olkowski, A.A. Response of turkey poults to soybean lectin levels typically encountered in commercial diets. 2. Effect on intestinal development and lymphoid organs. Poult. Sci. 2006, 85, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.; Freire, J.P.B.; Mourato, M.; Cabral, F.; Toullec, R.; Lallès, J.P. Comparative effects of different legume protein sources in weaned piglets: Nutrient digestibility, intestinal morphology and digestive enzymes. Livest. Prod. Sci. 2002, 74, 191–202. [Google Scholar] [CrossRef]
- Nakata, S.; Kimura, T. Effect of ingested toxic bean lectins on the gastrointestinal tract in the rat. J. Nutr. 1985, 115, 1621–1629. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, A.; Watt, W.B.; Stewart, J.C. A comprehensive scheme for the isolation of trypsin inhibitors and the agglutinin from soybean seeds. J. Agric. Food Chem. 1991, 39, 862–866. [Google Scholar] [CrossRef]
- Rouanet, J.M.; Besançon, P.; Lafont, J. Effect of lectins from leguminous seeds on rat duodenal enterokinase activity. Experientia 1983, 39, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology 1998, 115, 182–205. [Google Scholar] [CrossRef]
- Gordon, S.R.; Wood, M. Soybean agglutinin binding to corneal endothelial cell surfaces disrupts in situ monolayer integrity and actin organization and interferes with wound repair. Cell Tissue Res. 2009, 335, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, G.; Sun, Z.; Che, D.; Bao, N.; Zhang, X. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int. J. Mol. Sci. 2011, 12, 8502–8512. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- MacDermoit, R.P. Alterations of the mucosal immune system in inflammatory bowel disease. J. Gastroenterol. 1996, 31, 907–916. [Google Scholar] [CrossRef]
- Reisner, Y.; Sharon, N. 171 fractionation of subpopulations of mouse and human lymphocytes by peanut agglutinin or soybean agglutinin. In Methods in Enzymology; di Sabato, G., Langone, J.J., van Vunakis, H., Eds.; Academic Press: Cambridge, MA, USA, 1984; Volume 108, pp. 168–179. [Google Scholar]
- Krugluger, W.; Köller, M.; Allmaier, M.; Boltz-Nitulescu, G.; Förster, O. Ligation of N-acetylgalactosamine-containing structures on rat bone marrow cells enhances myeloid differentiation and murine granulocyte-macrophage colony-stimulating factor-induced proliferation. J. Leukocyte Biol. 1994, 55, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, C.F.; Figueiredo, R.C.; Henriques, M.G.M.O.; Barja-Fidalgo, C. Inflammatory and anti-inflammatory effects of soybean agglutinin. Braz. J. Med. Biol. Res. 1997, 30, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- García-Elorriaga, G.; del Rey-Pineda, G. Nutrition and intestinal microflora. J. Nutr. Ther. 2013, 2, 112–121. [Google Scholar] [CrossRef]
- Kim, J.; Choi, E.; Hong, Y.; Song, Y.; Han, J.; Lee, S.; Han, E.; Kim, T.; Choi, I.; Cho, K. Changes in korean adult females’ intestinal microbiota resulting from kimchi intake. J. Nutr. Food. Sci. 2016, 6, 2–11. [Google Scholar]
- Schulze, H.; Saini, H.S.; Huisman, J.; Hessing, M.; Van Den Berg, W.; Verstegen, M.W.A. Increased nitrogen secretion by inclusion of soya lectin in the diets of pigs. J. Sci. Food Agric. 1995, 69, 501–510. [Google Scholar] [CrossRef]
- Miao, S.; Zhao, C.; Zhu, J.; Hu, J.; Dong, X.; Sun, L. Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci. Rep. 2018, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ye, X.; Ng, T.B. Purification of melibiose-binding lectins from two cultivars of Chinese black soybeans. Acta Biochimica et Biophysica Sinica 2008, 40, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Mukhopadhyay, S.; Behera, B.; Bhol, C.S.; Dey, S.; Das, D.N.; Sinha, N.; Bissoyi, A.; Pramanik, K.; Maiti, T.K.; et al. Antitumor effect of soybean lectin mediated through reactive oxygen species-dependent pathway. Life Sci. 2014, 111, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Terashima, S.; Takano, Y.; Ohori, T.; Kanno, T.; Kimura, T.; Motoki, R.; Kawaguchi, T. Soybean agglutinin binding as a useful prognostic indicator in stomach cancer. Surg. Today 1997, 27, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Casañas Pimentel, R.G.; Robles Botero, V.; San Martín Martínez, E.; Gómez García, C.; Hinestroza, J.P. Soybean agglutinin-conjugated silver nanoparticles nanocarriers in the treatment of breast cancer cells. J. Biomater. Sci. Polym. Ed. 2016, 27, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Whitney, E.N.; Rolfes, S.R. Understanding Nutrition, 15th ed.; Cengage Learning Inc.: Boston, MA, USA, 2018. [Google Scholar]
- Veldman, A.; Veen, W.A.G.; Barug, D.; van Paridon, P.A. Effect of α-galactosides and α-galactosidase in feed on ileal piglet digestive physiology. J. Anim. Physiol. Anim. Nutr. 1993, 69, 57–65. [Google Scholar] [CrossRef]
- Zenhom, M.; Hyder, A.; de Vrese, M.; Heller, K.J.; Roeder, T.; Schrezenmeir, J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition Protein 3. J. Nutr. 2011, 141, 971–977. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, H.P.; Motherway, M.O.C.; Lakshminarayanan, B.; Stanton, C.; Paul Ross, R.; Brulc, J.; Menon, R.; O’Toole, P.W.; van Sinderen, D. Carbohydrate catabolic diversity of bifidobacteria and lactobacilli of human origin. Int. J. Food Microbiol. 2015, 203, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E. Prebiotics, probiotics, synbiotics and foods with regard to bone metabolism. In Nutritional Influences on Bone Health: 9th International Symposium; Weaver, C.M., Daly, R.M., Bischoff-Ferrari, H.A., Eds.; Springer International Publishing: Cham, Germany, 2016; pp. 153–167. [Google Scholar]
- Park, A.-R.; Oh, D.-K. Galacto-oligosaccharide production using microbial β-galactosidase: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, R.G.; Playne, M.J. Production, properties and applications of food-grade oligosaccharides. Trends Food Sci. Technol. 1996, 7, 353–361. [Google Scholar] [CrossRef]
- Leforestier, G.; Blais, A.; Blachier, F.; Marsset-Baglieri, A.; Davila-Gay, A.-M.; Perrin, E.; Tomé, D. Effects of galacto-oligosaccharide ingestion on the mucosa-associated mucins and sucrase activity in the small intestine of mice. Eur. J. Nutr. 2009, 48, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Veereman, G. Pediatric applications of inulin and oligofructose. J. Nutr. 2007, 137, 2585S–2589S. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Prabhu, P.N.; Benefiel, A.C.; Miller, M.J.; Chow, J.; Davis, S.R.; Gaskins, H.R. Galacto-oligosaccharides may directly enhance intestinal barrier function through the modulation of goblet cells. Mol. Nutr. Food Res. 2015, 59, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, S.; Hochi, S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. J. Mol. Med. 2010, 25, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, P.A.M.J.; Alliet, P.; Raes, M.; Alles, M.S.; Kroes, H.; Boehm, G.; Knippels, L.M.J.; Knol, J.; Vandenplas, Y. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J. Nutr. 2008, 138, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Gourbeyre, P.; Desbuards, N.; Grémy, G.; Le Gall, S.; Champ, M.; Denery-Papini, S.; Bodinier, M. Exposure to a galactooligosaccharides/inulin prebiotic mix at different developmental time points differentially modulates immune responses in mice. J. Agric. Food Chem. 2012, 60, 11942–11951. [Google Scholar] [CrossRef] [PubMed]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef] [PubMed]
- Ben, X.-M.; Li, J.; Feng, Z.-T.; Shi, S.-Y.; Lu, Y.-D.; Chen, R.; Zhou, X.-Y. Low level of galacto-oligosaccharide in infant formula stimulates growth of intestinal Bifidobacteria and Lactobacilli. World J. Gastroenterol. 2008, 14, 6564–6568. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Bauer, L.L.; Fahey, G.C.; Hogarth, A.J.C.L.; Wolf, B.W.; Hunter, D.E. Selected fructooligosaccharide (1-kestose, nystose, and 1f-β-fructofuranosylnystose) composition of foods and feeds. J. Agric. Food Chem. 1997, 45, 3076–3082. [Google Scholar] [CrossRef]
- Johnson-Henry, K.C.; Pinnell, L.J.; Waskow, A.M.; Irrazabal, T.; Martin, A.; Hausner, M.; Sherman, P.M. Short-chain fructo-oligosaccharide and inulin modulate inflammatory responses and microbial communities in caco2-bbe cells and in a mouse model of intestinal injury. J. Nutr. 2014, 144, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Schley, P.D.; Field, C.J. The immune-enhancing effects of dietary fibres and prebiotics. Br. J. Nutr. 2007, 87, S221–S230. [Google Scholar] [CrossRef]
- Rousseau, V.; Lepargneur, J.P.; Roques, C.; Remaud-Simeon, M.; Paul, F. Prebiotic effects of oligosaccharides on selected vaginal lactobacilli and pathogenic microorganisms. Anaerobe 2005, 11, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Zafar, T.A.; Weaver, C.M.; Zhao, Y.; Martin, B.R.; Wastney, M.E. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. J. Nutr. 2004, 134, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, T.; Wang, S.-E.; Wang, W.; Wang, Q.; Yu, H.-X. Fructo-oligosaccharides enhance the mineral absorption and counteract the adverse effects of phytic acid in mice. Nutrition 2010, 26, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; YongLie, C.; QianBing, W. Health benefit application of functional oligosaccharides. Carbohydr. Polym. 2009, 77, 435–441. [Google Scholar] [CrossRef]
- Spais, A.; Giannenas, I.; Florou-Paneri, P.; Christaki, E.; Botsoglou, N. Effect of the feed supplement bio-mos, a mannan-oligosaccharide, on the performance of broiler chickens (In Greek and English). J. Hell. Vet. Med. Soc. 2003, 54, 111–118. [Google Scholar]
- Sims, M.D.; Dawson, K.A.; Newman, K.E.; Spring, P.; Hoogell, D.M. Effects of dietary mannan oligosaccharide, bacitracin methylene disalicylate, or both on the live performance and intestinal microbiology of turkeys 1. Poult. Sci. 2004, 83, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Y. The Effects of Bio-Mos® on Intestinal Microflora and Immune Function of Juvenile Jian Carp (Cyprinus Carpio var. Jian). In Nutritional Biotechnology in the Feed and Food Industries, Proceedings of the Alltech’s 20th Annual Symposium (Suppl. 1—Abstracts of Posters Presented), Lexington, KY, USA, 22–26 May 2004; University of Kentucky: Lexington, KY, USA.
- Staykov, Y.; Spring, P.; Denev, S.; Sweetman, J. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss). Aquacult. Int. 2007, 15, 153–161. [Google Scholar] [CrossRef]
- Wismar, R.; Brix, S.; Frøkiær, H.; Lærke, H.N. Dietary fibers as immunoregulatory compounds in health and disease. Ann. N. Y. Acad. Sci. 2010, 1190, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Piao, X.S.; Kim, S.W.; Liu, P.; Wang, L.; Shen, Y.B.; Jung, S.C.; Lee, H.S. Effects of chito-oligosaccharide supplementation on performance, nutrient digestibility, and serum composition in broiler chickens. Poult. Sci. 2007, 86, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Dimitroglou, A.; Merrifield, D.L.; Moate, R.; Davies, S.J.; Spring, P.; Sweetman, J.; Bradley, G. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef] [PubMed]
- Spring, P.; Wenk, C.; Dawson, K.A.; Newman, K.E. The effects of dietary mannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks 1. Poult. Sci. 2000, 79, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Baurhoo, B.; Phillip, L.; Ruiz-Feria, C.A. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poult. Sci. 2007, 86, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.K.; Levitz, S.M. Fungal mannoproteins: The sweet path to immunodominance-mannose residues on glycoproteins trigger key host immune response that, if better harnessed, could protect against damage and disease. ASM News 2003, 69, 595–600. [Google Scholar]
- Wellens, A.; Garofalo, C.; Nguyen, H.; van Gerven, N.; Slättegård, R.; Hernalsteens, J.-P.; Wyns, L.; Oscarson, S.; De Greve, H.; Hultgren, S.; et al. Correction: Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH–oligomannose-3 complex. PLoS ONE 2008, 3, e2040. [Google Scholar] [CrossRef]
- Muanprasat, C.; Wongkrasant, P.; Satitsri, S.; Moonwiriyakit, A.; Pongkorpsakol, P.; Mattaveewong, T.; Pichyangkura, R.; Chatsudthipong, V. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: Mechanism of action and potential applications in intestinal disorders. Biochem. Pharmacol. 2015, 96, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Kwon, I.; Lohakare, J.; Heo, S.; Chae, B. Chito-oligosaccharides as an alternative to antimicrobials in improving performance, digestibility and microbial ecology of the gut in weanling pigs. Asian Aust. J. Anim. Sci. 2007, 20, 556. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Yin, Y.-L.; Nyachoti, C.M.; Huang, R.-L.; Li, T.-J.; Yang, C.; Yang, X.-j.; Gong, J.; Peng, J.; Qi, D.-S.; et al. Effect of dietary supplementation of chitosan and galacto-mannan-oligosaccharide on serum parameters and the insulin-like growth factor-I mRNA expression in early-weaned piglets. Domest. Anim. Endocrinol. 2005, 28, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Yoo, J.S.; Kim, H.J.; Lee, J.H.; Kim, I.H. Nutrient digestibility, blood profiles and fecal microbiota are influenced by chitooligosaccharide supplementation of growing pigs. Livest. Sci. 2009, 125, 298–303. [Google Scholar] [CrossRef]
- Liu, P.; Piao, X.S.; Kim, S.W.; Wang, L.; Shen, Y.B.; Lee, H.S.; Li, S.Y. Effects of chito-oligosaccharide supplementation on the growth performance, nutrient digestibility, intestinal morphology, and fecal shedding of Escherichia coli and Lactobacillus in weaning pigs1. J. Anim. Sci. 2008, 86, 2609–2618. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Piao, X.S.; Thacker, P.A.; Zeng, Z.K.; Li, P.F.; Wang, D.; Kim, S.W. Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K881. J. Anim. Sci. 2010, 88, 3871–3879. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, T.; Kawashima, K.; Nakayama, H.; Murakami, T.; Kanagawa, H.; Ishii, T.; Akiyama, K.; Yasuda, K.; Terada, F.; Kushibiki, S. Effect of cellooligosaccharide or synbiotic feeding on growth performance, fecal condition and hormone concentrations in Holstein calves. Anim. Sci. J. 2011, 82, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-S.; Li, J.-M.; Shi, B.; Liang, P.; Li, C. Optimization of reaction conditions for cello-oligosaccharides production from wheat straw by β-glucanase hydrolysis. Sci. Agric. Sin. 2013, 46, 2345–2352. [Google Scholar]
- Shang, Q.H.; Ma, X.K.; Li, M.; Zhang, L.H.; Hu, J.X.; Piao, X.S. Effects of α-galactosidase supplementation on nutrient digestibility, growth performance, intestinal morphology and digestive enzyme activities in weaned piglets. Anim. Feed Sci. Technol. 2018, 236, 48–56. [Google Scholar] [CrossRef]
- Jiao, L.F.; Song, Z.H.; Ke, Y.L.; Xiao, K.; Hu, C.H.; Shi, B. Cello-oligosaccharide influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim. Feed Sci. Technol. 2014, 195, 85–91. [Google Scholar] [CrossRef]
- Leeson, S.; Namkung, H.; Antongiovanni, M.; Lee, E.H. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005, 84, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jiao, L.F.; Xiao, K.; Luan, Z.S.; Hu, C.H.; Shi, B.; Zhan, X.A. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed Sci. Technol. 2013, 185, 175–181. [Google Scholar] [CrossRef]
- Drickamer, K. Multiplicity of lectin-carbohydrate interactions. Nat. Struct. Biol. 1995, 2, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Bhattacharyya, L.; Fant, J.; Macaluso, F.; Sabesan, S.; Brewer, C.F. Observation of unique cross-linked lattices between multiantennary carbohydrates and soybean lectin. Presence of pseudo-2-fold axes of symmetry in complex type carbohydrates. Biochemistry 1994, 33, 7495–7504. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, L.; Brewer, C.F. Formation of homogeneous carbohydrate–lectin cross-linked precipitates from mixtures of d-galactose/N-acetyl-d-galactosamine-specific lectins and multiantennary galactosyl carbohydrates. Eur. J. Biochem. 1992, 208, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.R.; Dessen, A.; Gupta, D.; Sabesan, S.; Sacchettini, J.C.; Brewer, C.F. X-ray crystallographic studies of unique cross-linked lattices between four isomeric biantennary oligosaccharides and soybean agglutinin. Biochemistry 1997, 36, 15073–15080. [Google Scholar] [CrossRef] [PubMed]
- Ujita, M.; Furukawa, K.; Aoki, N.; Sato, T.; Noda, A.; Nakamura, R.; Greenwalt, D.E.; Matsuda, T. A change in soybean agglutinin binding patterns of bovine milk fat globule membrane glycoproteins during early lactation. FEBS Lett. 1993, 332, 119–122. [Google Scholar] [CrossRef]
- Lis, H.; Sharon, N. Soybean agglutinin—A plant glycoprotein. Structure of the carboxydrate unit. J. Biol. Chem. 1978, 253, 3468–3476. [Google Scholar] [PubMed]
- Krugluger, W.; Lucas, T.; Köller, M.; Boltz-Nitulescu, G.; Förster, O. Soybean agglutinin binds a 160-kDa rat macrophage membrane glycoprotein and enhances cell differentiation and activation. Immunol. Lett. 1996, 52, 53–56. [Google Scholar] [CrossRef]
- Dam, T.K.; Brewer, C.F. Chapter 5—Multivalent lectin—Carbohydrate interactions: Energetics and mechanisms of binding. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 2010; Volume 63, pp. 139–164. [Google Scholar]
- Babot, J.D.; Argañaraz-Martínez, E.; Lorenzo-Pisarello, M.J.; Apella, M.C.; Perez Chaia, A. Cytotoxic damage of soybean agglutinin on intestinal epithelial cells of broiler chicks: In vitro protection by Bifidobacterium infantis CRL1395. FEMS Microbiol. Lett. 2016, 363, fnw114. [Google Scholar] [CrossRef] [PubMed]

| Types of Functional Oligosaccharide | Growth Performance | Intestinal Structure and Function | Immune System | Intestinal Microflora | References |
|---|---|---|---|---|---|
| Galacto-oligosaccharide | Increase the content of small intestinal mucosa-associated mucin and enterocyte-associated sucrase activity without modifying villi heights Modulate intestinal barrier function | Have a positive effect on mucosal immunity Prevents various immune pathologies such as allergies, autoimmune diseases, and inflammatory bowel disease | Protect against bacterial colonization and invasion by pathogens Enhance the growth or activity of some health-promoting microflora | [55,56,57,58,59,60,61,62,63] | |
| Fructo-oligosaccharide | Alter actin filament distribution Increase the intestinal barrier function Improve the activities of total protease and amylase | Modulate various parameters of the immune system Increase gut-associated lymphoid cells (including CD4+, CD8+, T-cells, B-cells, macrophages, and eosinophils) | Reduce pH Provide the production of gases and acids Stimulate the growth of probiotic-like bacteria | [64,65,66,67,68,69,70,71] | |
| Mannan-oligosaccharide | Increase body weight Improve weight gain and feed conversion | Improve intestinal morphology Modulate functions in the anterior and posterior intestinal regions Increase absorptive surface area Increase microvilli density and length | Offer a novel approach to support the microflora | [72,73,74,75,76,77,78,79,80,81,82] | |
| Chitosan oligosaccharide | Improve growth performance, nutrient digestibility, feed intake, serum composition, and microbial ecology | Increase villi heights and villus:crypt ratio Improve gut barrier function | Have anti-inflammatory, anti-oxidative, and antibacterial activities | Stimulate the populations of beneficial flora Decrease the number of harmful flora | [83,84,85,86,87,88,89,90] |
| Cello-oligosaccharide | Increase villi heights, villus height/crypt depth ratio, and villus surface area Increase the expression of brush border enzymes Increase nutrient transport systems Improve the barrier integrity | Improve the intestinal microflora | [91,92,93,94,95,96] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, L.; Farouk, M.H.; Qin, G.; Zhao, Y.; Bao, N. The Influences of Soybean Agglutinin and Functional Oligosaccharides on the Intestinal Tract of Monogastric Animals. Int. J. Mol. Sci. 2018, 19, 554. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020554
Pan L, Farouk MH, Qin G, Zhao Y, Bao N. The Influences of Soybean Agglutinin and Functional Oligosaccharides on the Intestinal Tract of Monogastric Animals. International Journal of Molecular Sciences. 2018; 19(2):554. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020554
Chicago/Turabian StylePan, Li, Mohammed Hamdy Farouk, Guixin Qin, Yuan Zhao, and Nan Bao. 2018. "The Influences of Soybean Agglutinin and Functional Oligosaccharides on the Intestinal Tract of Monogastric Animals" International Journal of Molecular Sciences 19, no. 2: 554. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020554







