Genome-Wide Identification and Expression Analysis of the UGlcAE Gene Family in Tomato
Abstract
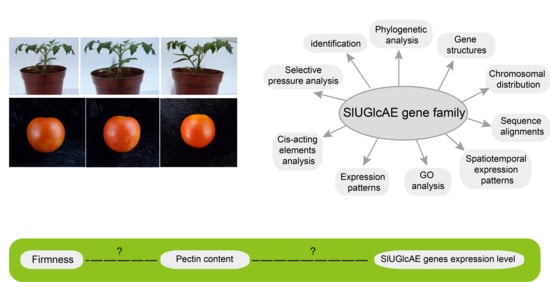
:1. Introduction
2. Results
2.1. The Identification of UGlcAE Gene Family in Tomato
2.2. Phylogenetic Analysis of the UGlcAE Genes in Tomato and Other Species
2.3. Structures of the UGlcAE Genes in the Tomato and Arabidopsis Thaliana
2.4. Chromosomal Distribution of the UGlcAE Genes in Tomato
2.5. Sequence Alignments and Hydrophilicity Analysis of SlUGlcAE Family
2.6. Spatiotemporal Expression Patterns Analysis of UGlcAE Genes in Tomato
2.7. GO Analysis of the UGlcAE Genes in the Tomato
2.8. Selective Pressure on UGlcAE Proteins in the Tomato
2.9. Cis-Acting Elements Analysis of the UGlcAE Genes in the Tomato
2.10. Expression Patterns of SlUGlcAE Family Genes in Response to IAA, GA and SA
2.11. The Firmness, Pectin Content and the Expression Level of UGlcAE Family Genes in Tomato Fruits at Different Development Stages
3. Discussion
4. Materials and Methods
4.1. Data Set Collection and Identification of SlUGlcAE Genes
4.2. Phylogenetic Analysis
4.3. Selective Pressure Analysis on UGlcAE Proteins in the Tomato
4.4. Chromosomal Location
4.5. Gene Structure Analysis
4.6. Gene Ontology Analysis
4.7. Analysis of Expression Profile of UGlcAE Genes in Tomato Various Tissues
4.8. Sequence Alignments and Prediction of Transmembrane Domains of SlUGlcAE Family
4.9. The Analysis of SlUGlcAE Family Protein Domains
4.10. Cis-Elements in the Upstream of SlUGlcAE Genes
4.11. Hormone Treatments
4.12. Plant Materials
4.13. Real-Time PCR
4.14. Fruit Firmness Measurement and Determination of Water-Soluble Pectin Content
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| UGlcAE/GAE | UDP-d-glucuronic acid 4-epimerase |
| IAA | indole-3-acetic acid |
| GA | gibberellin |
| SA | salicylic acid |
| GalA | d-galacturonic acid |
| UDP-GlcA | UDP-d-glucuronic acid |
| UDP-Glc | UDP-d-glucose |
| UDP-GalA | UDP-d-galacturonic acid |
| SDR | short-chain dehydrogenase/reductase |
| WSP | water-soluble pectin |
References
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, P.; McCann, M.C.; Roberts, K. Restricted cell elongation in arabidopsis hypocotyls is associated with a reduced average pectin esterification level. BMC Plant Biol. 2007, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Krupková, E.; Immerzeel, P.; Pauly, M.; Schmülling, T. The tumorous shoot development2 gene of arabidopsis encoding a putative methyltransferase is required for cell adhesion and co-ordinated plant development. Plant J. 2007, 50, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, T.W.; Der, J.P.; Honaas, L.A.; Depamphilis, C.W.; Anderson, C.T. Phylogenetic analysis of pectin-related gene families in physcomitrella patens and nine other plant species yields evolutionary insights into cell walls. BMC Plant Biol. 2014, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Mouille, G.; Ralet, M.C.; Cavelier, C.; Eland, C.; Effroy, D.; Hematy, K.; McCartney, L.; Truong, H.N.; Gaudon, V.; Thibault, J.F.; et al. Homogalacturonan synthesis in Arabidopsis thaliana requires a golgi-localized protein with a putative methyltransferase domain. Plant J. 2007, 50, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wages, C.J.; Davis, K.E.; Guyett, P.J.; Bar-Peled, M. Enzymatic characterization and comparison of various poaceae UDP-GlcA 4-epimerase isoforms. J. Biochem. 2009, 146, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C. Pectins and Their Manipulation; Seymour, G.B., Knox, J.P., Eds.; Blackwell Publishing: Oxford, UK, 2002; 262p, ISBN 1-841-27228-0. [Google Scholar]
- Loewus, F.; Chen, M.S.; Loewus, M.W. The myo-inositol oxidation pathway to cell wall polysaccharides *. Biogenes. Plant Cell Wall Polysacch. 1973, 1–27. [Google Scholar] [CrossRef]
- Tenhaken, R.; Thulke, O. Cloning of an enzyme that synthesizes a key nucleotide-sugar precursor of hemicellulose biosynthesis from soybean: UDP-glucose dehydrogenase. Plant Physiol. 1996, 112, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Orellana, A.; Mohnen, D. Enzymatic synthesis and purification of [(3)h]uridine diphosphate galacturonic acid for use in studying golgi-localized transporters. Anal. Biochem. 1999, 272, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Broach, B.; Gu, X.; Bar-Peled, M. Biosynthesis of UDP-glucuronic acid and UDP-galacturonic acid in Bacillus cereus subsp. Cytotoxis NVH 391-98. FEBS J. 2012, 279, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Usadel, B.; Schlüter, U.; Mølhøj, M.; Gipmans, M.; Verma, R.; Kossmann, J.; Reiter, W.D.; Pauly, M. Identification and characterization of a UDP-d-glucuronate 4-epimerase in arabidopsis. FEBS Lett. 2004, 569, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.; Schmandt, C. The biosynthesis of d-galacturonate in plants. Functional cloning and characterization of a membrane-anchored UDP-d-glucuronate 4-epimerase from arabidopsis. Plant Physiol. 2004, 135, 1221–1230. [Google Scholar]
- Gu, X.; Barpeled, M. The biosynthesis of UDP-galacturonic acid in plants. Functional cloning and characterization of arabidopsis UDP-d-glucuronic acid 4-epimerase. Plant Physiol. 2004, 136, 4256–4264. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Huang, J.; Gu, X.; Barpeled, M.; Xu, Y. Evolution of plant nucleotide-sugar interconversion enzymes. PLoS ONE 2011, 6, e27995. [Google Scholar] [CrossRef] [PubMed]
- Feingold, D.S.; Neufeld, E.F.; Hassid, W.Z. Enzymic synthesis of uridine diphosphate glucuronic acid and uridine diphosphate galacturonic acid with extracts from phaseolus aureus seedlings. Arch. Biochem. Biophys. 1958, 78, 401–406. [Google Scholar] [CrossRef]
- Gaunt, M.A.; Maitra, U.S.; Ankel, H. Uridine diphosphate galacturonate 4-epimerase from the blue-green alga anabaena flos-aquae. J. Biol. Chem. 1974, 249, 2366–2372. [Google Scholar] [PubMed]
- Ankel, H.; Tischer, R.G. UDP-d-glucuronate 4-epimerase in blue-green algae. Biochim. Biophys. Acta 1969, 178, 415–419. [Google Scholar] [CrossRef]
- Feingold, D.S.; Neufeld, E.F.; Hassid, W.Z. The 4-epimerization and decarboxylation of uridine diphosphate d-glucuronic acid by extracts from Phaseolus aureus seedlings. J. Biol. Chem. 1960, 235, 910. [Google Scholar] [PubMed]
- Dalessandro, G.; Northcote, D.H. Possible control sites of polysaccharide synthesis during cell growth and wall expansion of pea seedlings (Pisum sativum L.). Planta 1977, 134, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Dalessandro, G.; Northcote, D.H. Changes in enzymic activities of nucleoside diphosphate sugar interconversions during differentiation of cambium to xylem in sycamore and poplar. Biochem. J. 1977, 162, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Liljebjelke, K.; Adolphson, R.; Baker, K.; Doong, R.L.; Mohnen, D. Enzymatic synthesis and purification of uridine diphosphate [14C]galacturonic acid: A substrate for pectin biosynthesis. Anal. Biochem. 1995, 225, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Frirdich, E.; Whitfield, C. Characterization of glakp, a UDP-galacturonic acid C4-epimerase from Klebsiella pneumoniae with extended substrate specificity. J. Bacteriol. 2005, 187, 4104–4115. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Sun, Y.J.; Liu, M.; Li, L.N.; Kong, J.Q. cDNA isolation and functional characterization of UDP-d-glucuronic acid 4-epimerase family from Ornithogalum caudatum. Molecules 2016, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit development and ripening. Ann. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.G. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, A.; Yoshiyuki, O.; Kaori, I.; Kentaro, Y.; Hideki, N.; Eli, K.; Atsushi, T. Functional genomics of tomato in a post-genome-sequencing phase. Breed. Sci. 2013, 63, 14–20. [Google Scholar]
- Frelin, O.; Agrimi, G.; Laera, V.L.; Castegna, A.; Richardson, L.G.L.; Mullen, R.T.; Lermaortiz, C.; Palmieri, F.; Hanson, A.D. Identification of mitochondrial thiamin diphosphate carriers from arabidopsis and maize. Funct. Integr. Genom. 2012, 12, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Simons, C.; Firth, A.E.; Brown, C.M.; Hellens, R.P. Effect of 5′UTR introns on gene expression in Arabidopsis thaliana. BMC Genom. 2006, 7, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoden, J.B.; Hegeman, A.D.; Wesenberg, G.; Chapeau, M.C.; Frey, P.A.; Holden, H.M. Structural analysis of UDP-sugar binding to UDP-galactose 4-epimerase from Escherichia coli. Biochemistry 1997, 36, 6294–6304. [Google Scholar] [CrossRef] [PubMed]
- Oppermann, U.; Filling, C.; Hult, M.; Shafqat, N.; Wu, X.; Lindh, M.; Shafqat, J.; Nordling, E.; Kallberg, Y.; Persson, B.; et al. Short-chain dehydrogenases/reductases (SDR): The 2002 update. Chem.-Biol. Interact. 2003, 143–144, 247–253. [Google Scholar] [CrossRef]
- Wierenga, R.K.; Terpstra, P.; Hol, W.G.J. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J. Mol. Biol. 1986, 187, 101–107. [Google Scholar] [CrossRef]
- Wolters, H.; Jürgens, G. Survival of the flexible: Hormonal growth control and adaptation in plant development. Nat. Rev. Genet. 2009, 10, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Seif ElYazal, S.A.; Seif ElYazal, M.A.; Dwidar, E.F.; Rady, M.M. Phytohormone crosstalk research: Cytokinin and its crosstalk with other phytohormones. Curr. Protein Pept. Sci. 2015, 16, 395–405. [Google Scholar] [CrossRef]
- Wang, D.; Yeats, T.H.; Uluisik, S.; Rose, J.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Fernandezpozo, N.; Zheng, Y.; Snyder, S.; Nicolas, P.; Shinozaki, Y.; Fei, Z.; Catala, C.; Giovannoni, J.J.; Rose, J.K.; Mueller, L.A. The tomato expression atlas. Bioinformatics 2017, 33, 2397–2398. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.A.; Solow, T.H.; Taylor, N.; Skwarecki, B.; Buels, R.; Binns, J.; Lin, C.; Wright, M.H.; Ahrens, R.; Wang, Y. The sol genomics network: A comparative resource for solanaceae biology and beyond. Plant Physiol. 2005, 138, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Joung, J.G.; Tang, X.; Zheng, Y.; Huang, M.; Lee, J.M.; Mcquinn, R.; Tieman, D.M.; Alba, R.; Klee, H.J. Tomato functional genomics database: A comprehensive resource and analysis package for tomato functional genomics. Nucleic Acids Res. 2011, 39, D1156–D1163. [Google Scholar] [CrossRef] [PubMed]
- Lamesch, P.; Berardini, T.Z.; Li, D.; Swarbreck, D.; Wilks, C.; Sasidharan, R.; Muller, R.; Dreher, K.; Alexander, D.L.; Garcia-Hernandez, M.; et al. The arabidopsis information resource (tair): Improved gene annotation and new tools. Nucleic Acids Res. 2012, 40, D1202–D1210. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. Hmmer web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Milpetz, F.; Bork, P.; Ponting, C.P. Smart, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5857–5864. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Doronfaigenboim, A.; Stern, A.; Mayrose, I.; Bacharach, E.; Pupko, T. Selecton: A server for detecting evolutionary forces at a single amino-acid site. Bioinformatics 2005, 21, 2101–2103. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.; Doronfaigenboim, A.; Erez, E.; Martz, E.; Bacharach, E.; Pupko, T. Selecton 2007: Advanced models for detecting positive and purifying selection using a bayesian inference approach. Nucleic Acids Res. 2007, 35, W506–W511. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nielsen, R. Codon-substitution models for detecting molecular adaptation at individual sites along specific lineages. Mol. Biol. Evol. 2002, 19, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Bielawski, J.P.; Yang, Z.H. Accuracy and power of the likelihood ratio test in detecting adaptive molecular evolution. Mol. Biol. Evol. 2001, 18, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.; Zhang, H.; Luo, J.; Gao, G. Gsds 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Z.; Tong, X.; Chen, C.; Chen, M.; Meng, G.; Chen, P.; Li, C.; Xin, Y.; Gai, T. Genome-wide identification and characterization of fox genes in the silkworm, bombyx mori. Funct. Integr. Genom. 2015, 15, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L. Wego: A web tool for plotting go annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.Y.; Fraley, C.; Murua, A.; Raftery, A.E.; Ruzzo, W.L. Model-based clustering and data transformations for gene expression data. Bioinformatics 2001, 17, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; Von, H.G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. Plantcare, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Cai, X.; Ye, Z.; Li, H. Genome-wide identification and expression profiling analysis of trihelix gene family in tomato. Biochem. Biophys. Res. Commun. 2015, 468, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, G.; Zhang, J.; Zhang, Y.; Xie, Q.; Zhao, Z.; Pan, Y.; Hu, Z. The abiotic stress-responsive nac-type transcription factor SLNAC4 regulates salt and drought tolerance and stress-related genes in tomato (Solanum lycopersicum). Plant Cell Rep. 2014, 33, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, G.; Zhou, S.; Tu, Y.; Wang, Y.; Dong, T.; Hu, Z. A new tomato nac (NAM/ATAF1/2/CUC2) transcription factor, SLNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol. 2014, 55, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, R.; Tian, Y.; Han, H.; Xu, J.; Yao, Q. Genome-wide identification and analysis of the myb transcription factor superfamily in Solanum lycopersicum. Plant Cell Physiol. 2016, 57, 1657–1677. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, L.; Wang, A.; Xu, X.; Li, J. Ectopic overexpression of SLHsFA3, a heat stress transcription factor from tomato, confers increased thermotolerance and salt hypersensitivity in germination in transgenic arabidopsis. PLoS ONE 2013, 8, e54880. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Abbott, J.A. Firmness and force relaxation characteristics of tomato stored intact or as slices. Postharvest Biol. Technol. 2002, 24, 59–68. [Google Scholar] [CrossRef]
- Sandra, K.; Lynn, B.; Michael, N.; Spooner, D.M. Solanaceae—A model for linking genomics with biodiversity. Comp. Funct. Genom. 2004, 5, 285–291. [Google Scholar]
- Seymour, G.B.; Manning, K.; Eriksson, E.M.; Popovich, A.H.; King, G.J. Genetic identification and genomic organization of factors affecting fruit texture. J. Exp. Bot. 2002, 53, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B. Genomics meets horticulture. J. Hortic. Sci. Biotechnol. 2006, 81, 173. [Google Scholar] [CrossRef]










| No. | Gene Accession No. | NCBI Name | Chr. | Gene Name | Location | Arabidopsis Homologous | Size (AA) | ORF (bp) | Exon |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Solyc07g006220 | XP_010323223 | 7 | SlUGlcAE1 | ch07:1039601-1041900 | AT4G30440 | 425 | 1278 | 1 |
| 2 | Solyc12g010540 | XP_004251783 | 12 | SlUGlcAE1-like | ch12:3531001-3533400 | AT4G30440 | 432 | 1299 | 1 |
| 3 | Solyc03g083550 | XP_004234942 | 3 | SlUGlcAE2-like | ch03:53489301-53491700 | AT1G02000 | 406 | 1221 | 1 |
| 4 | Solyc01g091200 | XP_004229804 | 1 | SlUGlcAE3 | ch01:84887601-84890000 | AT4G00110 | 435 | 1308 | 1 |
| 5 | Solyc05g050990 | XP_004240046 | 5 | SlUGlcAE3-like1 | ch05:61200401-61202800 | AT4G00110 | 435 | 1308 | 1 |
| 6 | Solyc10g018260 | XP_004248561 | 10 | SlUGlcAE3-like2 | ch10:7314201-7316600 | AT4G00110 | 435 | 1308 | 1 |
| 7 | Solyc08g079440 | XP_004245716 | 8 | SlUGlcAE5 | ch08:62963401-62965900 | AT4G12250 | 445 | 1338 | 1 |
| 8 | Solyc09g092330 | XP_004247883 | 9 | SlUGlcAE6 | ch09:71458501-71461000 | AT3G23820 | 452 | 1359 | 1 |
| 9 | Solyc05g053790 | XP_004239852 | 5 | SlUGlcAE6-like | ch05:63814001-63816400 | AT3G23820 | 433 | 1302 | 1 |
| Primer Name | Sense Sequence (5′ → 3′) | Antisense Sequence (5′ → 3′) |
|---|---|---|
| EF1a | TACTGGTGGTTTTGAAGCTG | AACTTCCTTCACGATTTCATCATA |
| CAC | CCTCCGTTGTGATGTAACTGG | ATTGGTGGAAAGTAACATCATCG |
| SlUGlcAE1 | TGTAAAATGGCTAATCCACAACCT | AAAAACCGCAATCCAGTAATCG |
| SlUGlcAE1-like | ACCGGTGTTTCGCTTCAACGAGT | AAGACTACCCCATGTGGAGGAGAG |
| SlUGlcAE2-like | GCGAGTCTATACGCTGCCACA | CGTCTTCTTACCACCACTTCCTG |
| SlUGlcAE3 | CAACCCCAGGAAAGTTCAAGATGG | GACGAAGAAGCTGGAGATCTGTAG |
| SlUGlcAE3-like1 | AGGCAGCTAATCATGGCACAGTC | AAGATCAGATACCGGGACAGGTG |
| SlUGlcAE3-like2 | TCATGGGACTGTTGCTAGGGACT | CCTTGGCAACTTCATCACAGCTC |
| SlUGlcAE5 | TGTAAAATGGCTAATCCACAACCT | AAAACCGCAATCCAGTAATCG |
| SlUGlcAE6 | CCACCTGACACAAGCAAAACCAC | GGAGGATAGAAGGTTATGGGTAGTGG |
| SlUGlcAE6-like | GGACTGATCAACCAGCTAGTCTC | CGTAAACCTTGATCGGCTTCCCTTG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Li, J.; Pan, Y.; Zhang, Y.; Ni, L.; Wang, Y.; Zhang, X. Genome-Wide Identification and Expression Analysis of the UGlcAE Gene Family in Tomato. Int. J. Mol. Sci. 2018, 19, 1583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061583
Ding X, Li J, Pan Y, Zhang Y, Ni L, Wang Y, Zhang X. Genome-Wide Identification and Expression Analysis of the UGlcAE Gene Family in Tomato. International Journal of Molecular Sciences. 2018; 19(6):1583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061583
Chicago/Turabian StyleDing, Xing, Jinhua Li, Yu Pan, Yue Zhang, Lei Ni, Yaling Wang, and Xingguo Zhang. 2018. "Genome-Wide Identification and Expression Analysis of the UGlcAE Gene Family in Tomato" International Journal of Molecular Sciences 19, no. 6: 1583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19061583