Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes
Abstract
:1. Introduction
2. Results
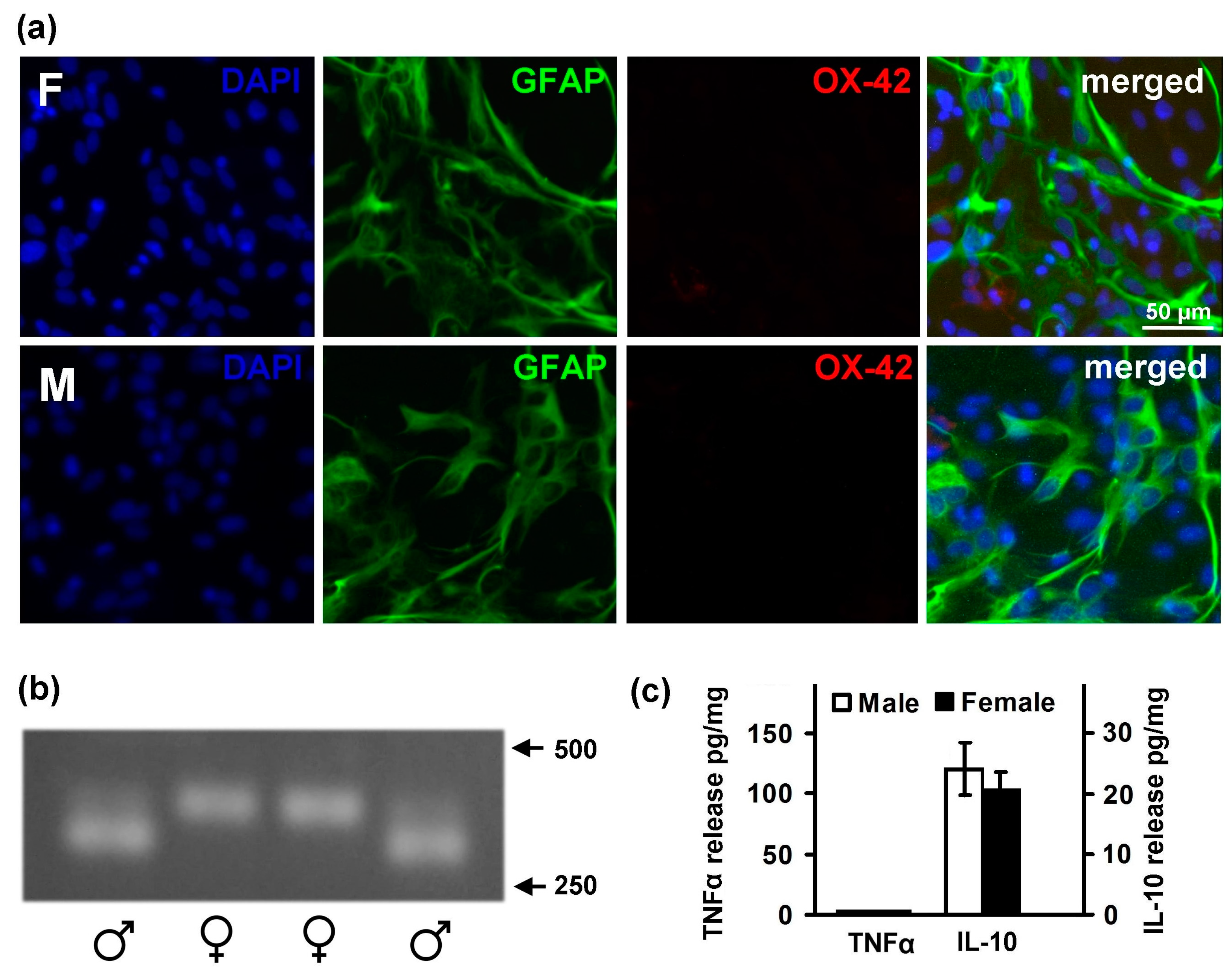
2.1. Astrocytes Isolated from Male or Female Pups Reveal Similar Morphology, But Demonstrate Differences in Responses to LPS
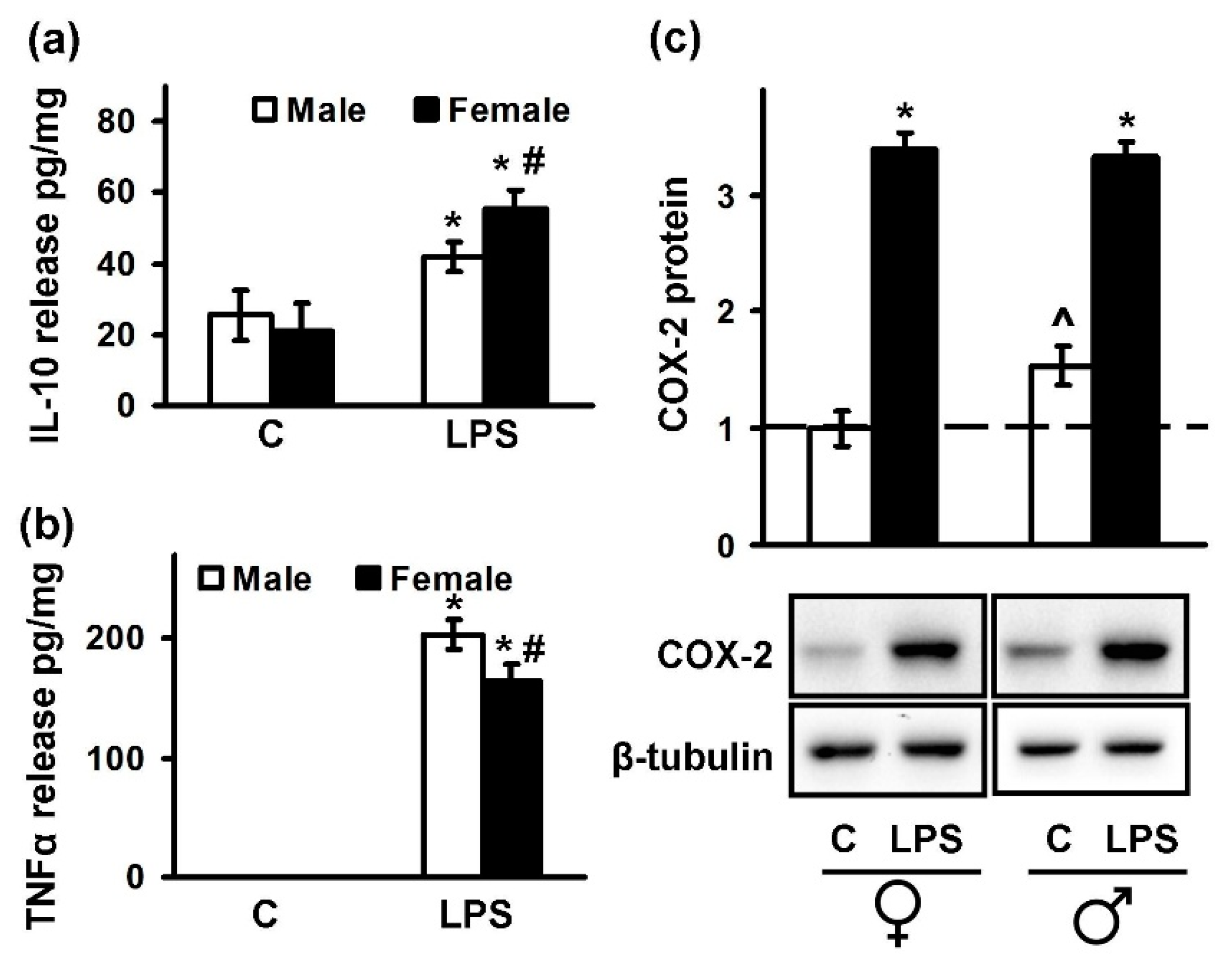
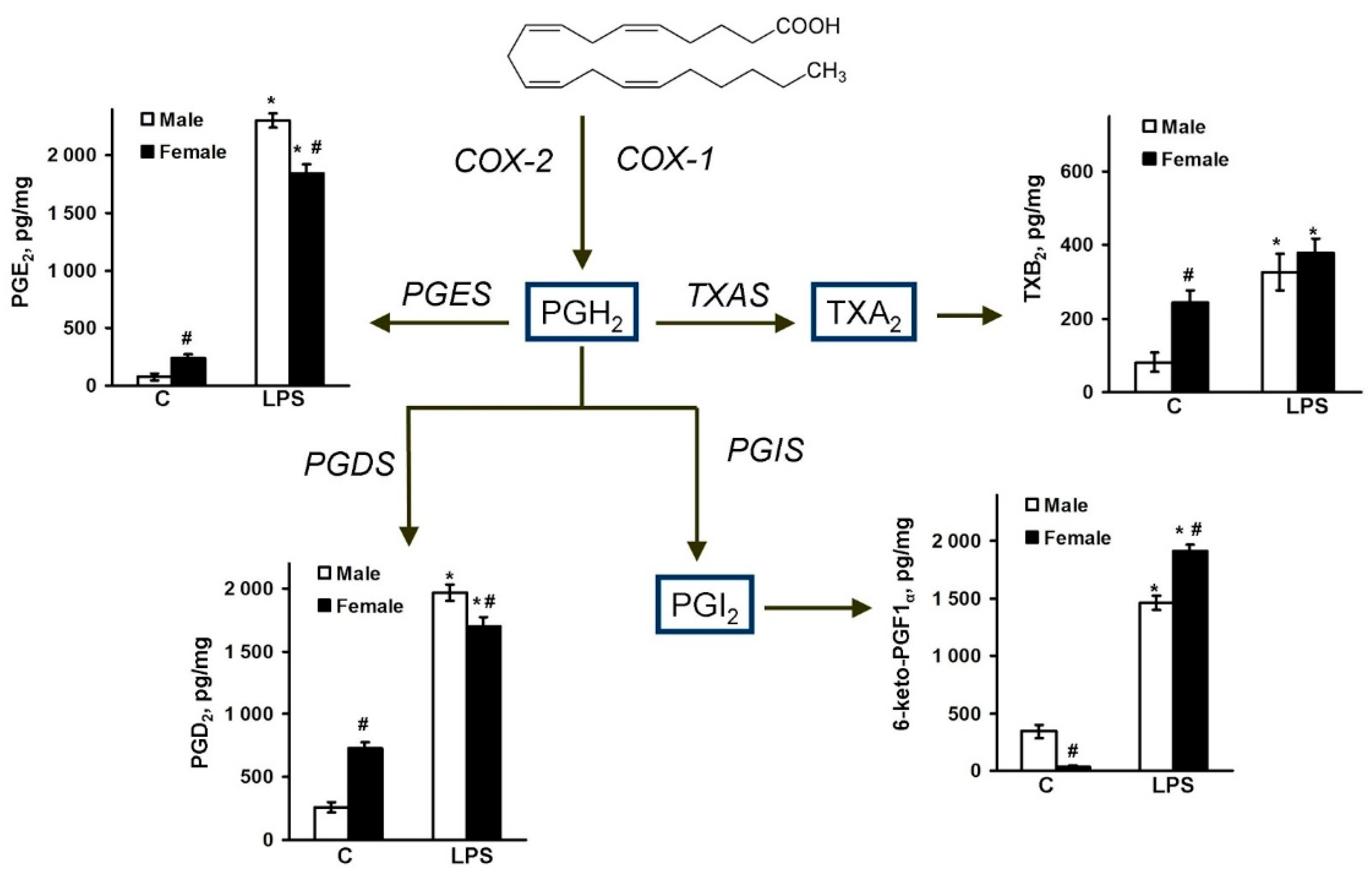
2.2. Sex-Mediated Differences between Male and Female LPS-Triggered Release of Prostaglandins
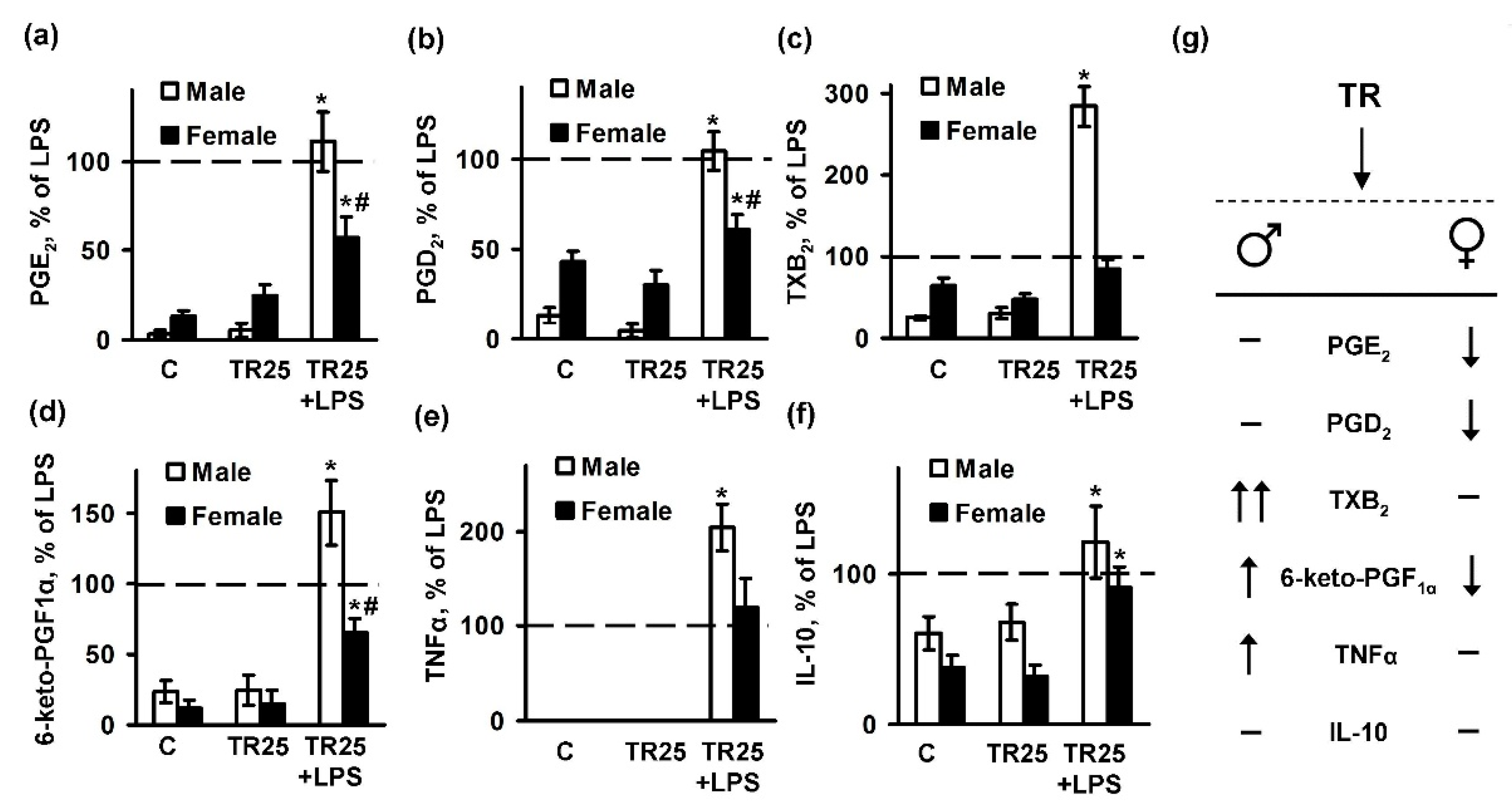
2.3. Sex Differences in an Inflammatory Response of Astrocytes Treated with Trilostan
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Primary Cell Culture
4.3. Rat Genotyping
4.4. Western Blot Analysis
4.5. Immunofluorescence Analysis
4.6. UPLC-MS/MS Conditions and Sample Preparation
4.7. Determination of TNFα and IL-10 by Enzyme-Linked Immunoassay
4.8. Experimental Data Analysis and Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TLR | toll-like receptor |
| LPS | lipopolysaccharide |
| TNFα | tumor necrosis factor alpha |
| IL-10 | interleukin-10 |
| TR | trilostane |
| COX-2 | cyclooxygenase-2 |
| 3β-HSD | 3β-Hydroxysteroid dehydrogenase |
References
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, S.; Rossi, A.; Krauth, V.; Dehm, F.; Troisi, F.; Bilancia, R.; Weinigel, C.; Rummler, S.; Werz, O.; Sautebin, L. Sex differences in prostaglandin biosynthesis in neutrophils during acute inflammation. Sci. Rep. 2017, 7, 3759. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.H.; Lenz, K.M. The immune system as a novel regulator of sex differences in brain and behavioral development. J. Neurosci. Res. 2017, 95, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Cosimo Melcangi, R.; Garcia-Segura, L.M. Sex-specific therapeutic strategies based on neuroactive steroids: In search for innovative tools for neuroprotection. Horm. Behav. 2010, 57, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astiz, M.; Acaz-Fonseca, E.; Garcia-Segura, L.M. Sex differences and effects of estrogenic compounds on the expression of inflammatory molecules by astrocytes exposed to the insecticide dimethoate. Neurotox. Res. 2014, 25, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Ezio, G.; Giancarlo, P. Sex and Gender Differences in the Brain Cholinergic System and in the Response to Therapy of Alzheimer Disease with Cholinesterase Inhibitors. Curr. Alzheimer Res. 2018, 15. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Bilbo, S.D. Sex, glia, and development: Interactions in health and disease. Horm. Behav. 2012, 62, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strokin, M.; Sergeeva, M.; Reiser, G. Role of Ca2+-independent phospholipase A2 and n-3 polyunsaturated fatty acid docosahexaenoic acid in prostanoid production in brain: Perspectives for protection in neuroinflammation. Int. J. Dev. Neurosci. 2004, 22, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Arbo, B.D.; Bennetti, F.; Ribeiro, M.F. Astrocytes as a target for neuroprotection: Modulation by progesterone and dehydroepiandrosterone. Prog. Neurobiol. 2016, 144, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Loram, L.C.; Sholar, P.W.; Taylor, F.R.; Wiesler, J.L.; Babb, J.A.; Strand, K.A.; Berkelhammer, D.; Day, H.E.W.; Maier, S.F.; Watkins, L.R. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 2012, 37, 1688–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahimian, R.; Cordeau, P.; Kriz, J. Brain Response to Injuries: When Microglia Go Sexist. Neuroscience 2018. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Steelman, A.J.; Koito, H.; Li, J. Astrocytes promote TNF-mediated toxicity to oligodendrocyte precursors. J. Neurochem. 2011, 116, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.; Azbukina, N.; Lopachev, A.; Kulichenkova, K.; Astakhova, A.; Sergeeva, M. Rosiglitazone as a Modulator of TLR4 and TLR3 Signaling Pathways in Rat Primary Neurons and Astrocytes. Int. J. Mol. Sci. 2018, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Pankevich, E.V.; Astakhova, A.A.; Chistyakov, D.V.; Sergeeva, M.G. Antiinflammatory effect of rosiglitazone via modulation of mRNA stability of interleukin 10 and cyclooxygenase 2 in astrocytes. Biochemistry 2017, 82, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; Salas-Perdomo, A.; Marquez-Kisinousky, L.; Santalucia, T.; Planas, A.M.; Salas-Pérdomo, A.; Márquez-Kisinousky, L.; et al. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J. Biol. Chem. 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chalimoniuk, M.; Shu, Y.; Simonyi, A.; Sun, A.Y.; Gonzalez, F.A.; Weisman, G.A.; Wood, W.G.; Sun, G.Y. Prostaglandin E2 production in astrocytes: Regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Grabeklis, S.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G.; Reiser, G. Astrocytes synthesize primary and cyclopentenone prostaglandins that are negative regulators of their proliferation. Biochem. Biophys. Res. Commun. 2018, 39, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M. Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology 2018, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F. Intracrinology. Mol. Cell. Endocrinol. 1991, 78, C113–C118. [Google Scholar] [CrossRef]
- Zwain, I.H.; Yen, S.S.C. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology 1999, 140, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.M.; Melcangi, R.C. Steroids and glial cell function. Glia 2006, 54, 485–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acaz-Fonseca, E.; Avila-Rodriguez, M.; Garcia-Segura, L.M.; Barreto, G.E. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog. Neurobiol. 2016, 144, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Potts, G.O.; Creange, J.E.; Harding, H.R.; Schane, H.P. Trilostane, an orally active inhibitor of steroid biosynthesis. Steroids 1978, 32, 257–267. [Google Scholar] [CrossRef]
- Buyanova, S.M.; Chistyakov, D.V.; Astakhova, A.A.; Sergeeva, M.G. The effect of dehydroepiandrosterone on inflammatory response of astroglial cells. Biochem. Suppl. Ser. A Membr. Cell Biol. 2017, 11, 304–310. [Google Scholar] [CrossRef]
- Fuente-Martin, E.; Garcia-Caceres, C.; Morselli, E.; Clegg, D.J.; Chowen, J.A.; Finan, B.; Brinton, R.D.; Tschöp, M.H. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev. Endocr. Metab. Disord. 2013, 14, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, S.; Leone, M.G.; Grima, J.; Silvestrini, B.; Cheng, C.Y. Astrocytes synthesize and secrete prostaglandin D synthetase in vitro. Biochim. Biophys. Acta 1996, 1310, 269–276. [Google Scholar] [CrossRef]
- Czlonkowska, A.; Ciesielska, A.; Gromadzka, G.; Kurkowska-Jastrzebska, I. Estrogen and cytokines production—The possible cause of gender differences in neurological diseases. Curr. Pharm. Des. 2005, 11, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Astakhova, A.A.; Chistyakov, D.V.; Pankevich, E.V.; Sergeeva, M.G. Regulation of cyclooxygenase 2 expression by agonists of PPAR nuclear receptors in the model of endotoxin tolerance in astrocytes. Biochemistry 2015, 80, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Van Noort, J.M.; Bsibsi, M. Toll-like receptors in the CNS: Implications for neurodegeneration and repair. Prog. Brain Res. 2009, 175, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.A.; Duncan, D.S.; Miller, S.D. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav. Immun. 2008, 22, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Galindo, M.; Acaz-Fonseca, E.; Bellini, M.J.; Garcia-Segura, L.M. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol. Sex Differ. 2011, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Guan, P.-P.; Yu, X.; Zhang, L.-C.; Su, Y.-N.; Wang, Z.-Y. Prostaglandin I2 Attenuates Prostaglandin E2-Stimulated Expression of Interferon γ in a β-Amyloid Protein- and NF-κB-Dependent Mechanism. Sci. Rep. 2016, 6, 20879. [Google Scholar] [CrossRef] [PubMed]
- Espallergues, J.; Givalois, L.; Temsamani, J.; Laruelle, C.; Maurice, T. The 3β-hydroxysteroid dehydrogenase inhibitor trilostane shows antidepressant properties in mice. Psychoneuroendocrinology 2009, 34, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Amateur, S.K.; McCarthy, M.M. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J. Neuroendocrinol. 2002, 14, 904–910. [Google Scholar] [CrossRef]
- Mong, J.A.; McCarthy, M.M. Steroid-induced developmental plasticity in hypothalamic astrocytes: Implications for synaptic patterning. J. Neurobiol. 1999, 40, 602–619. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Segura, L.M.; Suarez, I.; Segovia, S.; Tranque, P.A.; Calés, J.M.; Aguilera, P.; Olmos, G.; Guillamón, A. The distribution of glial fibrillary acidic protein in the adult rat brain is influenced by the neonatal levels of sex steroids. Brain Res. 1988, 456, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, J.M.; Sholar, P.W.; Bilbo, S.D. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012, 120, 948–963. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Wright, C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 402–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crain, J.M.; Nikodemova, M.; Watters, J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013, 91, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundar Boyalla, S.; Barbara Victor, M.; Roemgens, A.; Beyer, C.; Arnold, S. Sex- and brain region-specific role of cytochrome c oxidase in 1-methyl-4-phenylpyridinium-mediated astrocyte vulnerability. J. Neurosci. Res. 2011, 89, 2068–2082. [Google Scholar] [CrossRef] [PubMed]
- Chistyakov, D.V.; Astakhova, A.A.; Sergeeva, M.G. Resolution of inflammation and mood disorders. Exp. Mol. Pathol. 2018, 105, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Achiron, A.; Gurevich, M. Gender effects in relapsing-remitting multiple sclerosis: Correlation between clinical variables and gene expression molecular pathways. J. Neurol. Sci. 2009, 286, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Golden, L.C.; Voskuhl, R. The importance of studying sex differences in disease: The example of multiple sclerosis. J. Neurosci. Res. 2017, 95, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Jobin, C.; Larochelle, C.; Parpal, H.; Coyle, P.K.; Duquette, P. Gender issues in multiple sclerosis: An update. Women’s Health 2010, 6, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Weill-Engerer, S.; Liere, P.; Robert, F.; Franklin, R.J.M.; Garcia-Segura, L.M.; Lambert, J.J.; Mayo, W.; Melcangi, R.C.; Parducz, A.; et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog. Neurobiol. 2003, 71, 3–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robel, P.; Young, J.; Corpéchot, C.; Mayo, W.; Perché, F.; Haug, M.; Simon, H.; Baulieu, E.E. Biosynthesis and assay of neurosteroids in rats and mice: Functional correlates. J. Steroid Biochem. Mol. Biol. 1995, 53, 355–360. [Google Scholar] [CrossRef]
- Young, J.; Corpéchot, C.; Perché, F.; Eychenne, B.; Haug, M.; Baulieu, E.E.; Robel, P. Neurosteroids in the mouse brain: Behavioral and pharmacological effects of a 3β-hydroxysteroid dehydrogenase inhibitor. Steroids 1996, 61, 144–149. [Google Scholar] [CrossRef]
- Lüttgenau, J.; Herzog, K.; Strüve, K.; Latter, S.; Boos, A.; Bruckmaier, R.M.; Bollwein, H.; Kowalewski, M.P. LPS-mediated effects and spatio-temporal expression of TLR2 and TLR4 in the bovine corpus luteum. Reproduction 2016, 151, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chistyakov, D.V.; Aleshin, S.; Sergeeva, M.G.; Reiser, G. Regulation of peroxisome proliferator-activated receptor β/δ expression and activity levels by toll-like receptor agonists and MAP kinase inhibitors in rat astrocytes. J. Neurochem. 2014, 130, 563–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chistyakov, D.V.; Aleshin, S.E.; Astakhova, A.A.; Sergeeva, M.G.; Reiser, G. Regulation of peroxisome proliferator-activated receptors (PPAR) α and -γ of rat brain astrocytes in the course of activation by toll-like receptor agonists. J. Neurochem. 2015, 134, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truett, G.E.; Heeger, P.; Mynatt, R.L.; Truett, A.A.; Walker, J.A.; Warman, M.L. Preparation of PCR-quality mouse genomic dna with hot sodium hydroxide and tris (HotSHOT). Biotechniques 2000, 29, 52–54. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chistyakov, D.V.; Azbukina, N.V.; Astakhova, A.A.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes. Int. J. Mol. Sci. 2018, 19, 2793. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092793
Chistyakov DV, Azbukina NV, Astakhova AA, Goriainov SV, Chistyakov VV, Sergeeva MG. Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes. International Journal of Molecular Sciences. 2018; 19(9):2793. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092793
Chicago/Turabian StyleChistyakov, Dmitry V., Nadezda V. Azbukina, Alina A. Astakhova, Sergei V. Goriainov, Viktor V. Chistyakov, and Marina G. Sergeeva. 2018. "Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes" International Journal of Molecular Sciences 19, no. 9: 2793. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092793




