Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway
Abstract
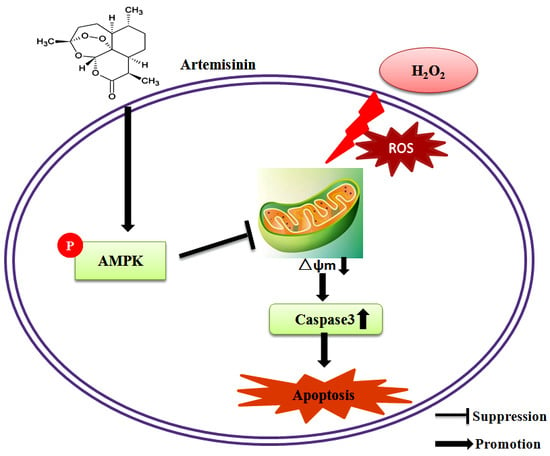
:1. Introduction
2. Results
2.1. Artemisinin Attenuated the Cell Viability Induced by H2O2 in SH-SY5Y Cells
2.2. Artemisinin Pretreatment Attenuated H2O2-Induced Apoptosis in SH-SY5Y Cells
2.3. Artemisinin Inhibited H2O2-Induced Increase in ROS Level and Restored the Mitochondrial Membrane Potential in SH-SY5Y Cells
2.4. Artemisinin Stimulated the Phosphorylation of AMPK in SH-SY5Y Cells
2.5. AMPK Mediated the Protective Effects of Artemisinin in SH-SY5Y Cells
2.6. Artemisinin Protected Primary Cultured Hippocampal Neurons Against H2O2 Induced Injury via AMPK Kinase
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. SH-SY5Y Cell Culture
4.2.2. Hippocampal Neurons Culture
4.2.3. MTT Assay
4.2.4. Hoechst 33258 Staining
4.2.5. Annexin V-FITC/PI Staining for Apoptosis Evaluation
4.2.6. Measurement of Intracellular ROS Levels
4.2.7. Measurement of Mitochondrial Membrane Potential (∆ψm)
4.2.8. Immunocytochemistry (ICC)
4.2.9. TUNEL Assay
4.2.10. Caspase-3 Activity Assay
4.2.11. Western Blotting
4.2.12. Transfection of ShRNA Plasmid
4.2.13. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| H2O2 | Hydrogen peroxide |
| AMPK | Adenosine Monophosphate -activated protein kinase |
| DMSO | Dimethyl sulfoxide |
| FBS | Fatalbovineserum |
| AD | Alzheimer’s disease |
| BCA | Bicinchoninic acid |
| RIPA | Radioimmunoprecipitation assay |
| shAMPK | AMP-activated protein kinase knockdown plasmid |
| Compound C | Adenosine Monophosphate -activated protein kinase inhibitor |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanine iodide |
| ICC | Immunocytochemistry analysis |
| NeuN | Neuronal nuclear protein |
References
- Ferrini, M.G.; Gonzalez-Cadavid, N.F.; Rajfer, J. Aging related erectile dysfunction—potential mechanism to halt or delay its onset. Transl. Androl. Urol. 2017, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Tezil, T.; Basaga, H. Modulation of cell death in age-related diseases. Curr. Pharm. Des. 2014, 20, 3052–3067. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Iglesias, R.C.; Ruiz, R.; Aparicio, S.; Crespo, J.; Lopez, L.D.; Manna, P.P.; Giampieri, F.; Battino, M. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol. Res. 2018, 131, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Su, J.H.; Ivins, K.J.; Van Houten, B.; Cotman, C.W. Bcl-2 facilitates recovery from DNA damage after oxidative stress. Exp. Neurol. 1999, 159, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Gorman, A.; McGowan, A.; O’Neill, C.; Cotter, T. Oxidative stress and apoptosis in neurodegeneration. J. Neurol. Sci. 1996, 139, 45–52. [Google Scholar] [CrossRef]
- Hsuuw, Y.D.; Chang, C.K.; Chan, W.H.; Yu, J.S. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell. Physiol. 2005, 205, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, M.; Afrin, S.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Amici, A.; Battino, M.; Giampieri, F. Protective effects of Manuka honey on LPS-treated RAW 264.7 macrophages. Part 2: Control of oxidative stress induced damage, increase of antioxidant enzyme activities and attenuation of inflammation. Food Chem. Toxicol. 2018, 120, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Whittemore, E.; Loo, D.; Watt, J.; Cotmans, C. A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neuroscience 1995, 67, 921–932. [Google Scholar] [CrossRef]
- Lindenboim, L.; Haviv, R.; Stein, R. Bcl-xL inhibits different apoptotic pathways in rat PC12 cells. Neurosci. Lett. 1998, 253, 37–40. [Google Scholar] [CrossRef]
- Tu, Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nature Med. 2011, 17, 1217. [Google Scholar] [CrossRef]
- Burkewitz, K.; Zhang, Y.; Mair, W.B. AMPK at the nexus of energetics and aging. Cell Metab. 2014, 20, 10–25. [Google Scholar] [CrossRef]
- Steely, A.M.; Willoughby, J.A.; Sundar, S.N.; Aivaliotis, V.I.; Firestone, G.L. Artemisinin disrupts androgen responsiveness of human prostate cancer cells by stimulating the 26S proteasome-mediated degradation of the androgen receptor protein. Anti-cancer Drugs 2017, 28, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Schmuck, G.; Roehrdanz, E.; Haynes, R.K.; Kahl, R. Neurotoxic mode of action of artemisinin. Antimicrob. Agents Chemother. 2002, 46, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.M.; Xu, F.-h.; Fady, C.; Jacoby, F.J.; Duffey, D.C.; Tu, Y.; Lichtenstein, A. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic. Biol. Med. 1997, 22, 73–83. [Google Scholar] [CrossRef]
- Zeng, Z.; Xu, J.; Zheng, W. Artemisinin protects PC12 cells against β-amyloid-induced apoptosis through activation of the ERK1/2 signaling pathway. Redox Biol. 2017, 12, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Yagi, Y.; Nakano, O.; Hashimoto, T.; Kimura, K.; Asakawa, Y.; Zhong, M.; Narimatsu, S.; Gohda, E. Induction of neurite outgrowth in PC12 cells by artemisinin through activation of ERK and p38 MAPK signaling pathways. Brain Res. 2013, 1490, 61–71. [Google Scholar]
- Zheng, W.; Chong, C.-M.; Wang, H.; Zhou, X.; Zhang, L.; Wang, R.; Meng, Q.; Lazarovici, P.; Fang, J. Artemisinin conferred ERK mediated neuroprotection to PC12 cells and cortical neurons exposed to sodium nitroprusside-induced oxidative insult. Free Radic. Biol. Med. 2016, 97, 158–167. [Google Scholar] [CrossRef]
- Zuo, S.; Li, Q.; Liu, X.; Feng, H.; Chen, Y. The potential therapeutic effects of artesunate on stroke and other central nervous system diseases. BioMed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nature Rev. Mol. Cell Biol. 2012, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Alvarez-Suarez, J.M.; Cordero, M.D.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Santos-Buelga, C.; González-Paramás, A.M.; Astolfi, P.; Rubini, C. Strawberry consumption improves aging-associated impairments, mitochondrial biogenesis and functionality through the AMP-Activated Protein Kinase signaling cascade. Food Chem. 2017, 234, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, X.; Wang, Y.; Liu, Y.; Xia, M. Supplementation of Cyanidin-3-O-β-Glucoside Promotes Endothelial Repair and Prevents Enhanced Atherogenesis in Diabetic Apolipoprotein E–Deficient Mice–3. J. Nutr. 2013, 143, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Reznick, R.M.; Zong, H.; Li, J.; Morino, K.; Moore, I.K.; Hannah, J.Y.; Liu, Z.-X.; Dong, J.; Mustard, K.J.; Hawley, S.A. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007, 5, 151–156. [Google Scholar] [CrossRef]
- Martín, D.; Salinas, M.; Fujita, N.; Tsuruo, T.; Cuadrado, A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J. Biol. Chem. 2002, 277, 42943–42952. [Google Scholar] [CrossRef]
- Kahn, B.B.; Alquier, T.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005, 1, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, R.A.; Birnbaum, M.J. An energetic tale of AMPK-independent effects of metformin. J. Clin. Investig. 2010, 120, 2267–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffetin, A.; Bruneel, F.; Roussel, C.; Thellier, M.; Buffet, P.; Caumes, E.; Jauréguiberry, S. Use of artesunate in non-malarial indications. Med. Mal. Infect. 2018, 48, 238–249. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, Z.; Huang, G.; Wang, N.; Wang, E.; Guo, Q. Sevoflurane post-conditioning enhanced hippocampal neuron resistance to global cerebral ischemia induced by cardiac arrest in rats through PI3K/Akt survival pathway. Front. Cell. Neurosci. 2016, 10, 271. [Google Scholar] [CrossRef]
- Chong, C.-M.; Zheng, W. Artemisinin protects human retinal pigment epithelial cells from hydrogen peroxide-induced oxidative damage through activation of ERK/CREB signaling. Redox Biol. 2016, 9, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Cavallucci, V.; D’Amelio, M.; Cecconi, F. Aβ toxicity in Alzheimer’s disease. Mol. Neurobiol. 2012, 45, 366–378. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogenesis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Kumar, S.; Vijayan, M.; Bhatti, G.K.; Reddy, P.H. Therapeutic strategies for mitochondrial dysfunction and oxidative stress in age-related metabolic disorders. Prog. Mol. Biol. Transl. Sci. 2017, 146, 13–46. [Google Scholar] [PubMed]
- Han, X.; Tai, H.; Wang, X.; Wang, Z.; Zhou, J.; Wei, X.; Ding, Y.; Gong, H.; Mo, C.; Zhang, J. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD+ elevation. Aging Cell 2016, 15, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Adegoke, O.O.; Qiao, F.; Liu, Y.; Longley, K.; Feng, S.; Wang, H. Overexpression of Ubiquilin-1 Alleviates Alzheimer’s Disease-Caused Cognitive and Motor Deficits and Reduces Amyloid-beta Accumulation in Mice. J. Alzheimer’s Dis. JAD 2017, 59, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-P.; Li, W.; Liu, R.; Yang, S. Artemisinin Prevents Glutamate-Induced Neuronal Cell Death Via Akt Pathway Activation. Front. Cell. Neurosci. 2018, 12, 108. [Google Scholar] [CrossRef] [Green Version]
- Karbwang, J.; Sukontason, K.; Rimchala, W.; Namsiripongpun, W.; Tin, T.; Auprayoon, P.; Tumsupapong, S.; Bunnag, D.; Harinasuta, T. Preliminary report: A comparative clinical trial of artemether and quinine in severe falciparum malaria. Southeast Asian J. Trop. Med. Public Health 1992, 23, 768. [Google Scholar]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Fang, J.; Li, S.; Gaur, U.; Xing, X.; Wang, H.; Zheng, W. Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway. Int. J. Mol. Sci. 2019, 20, 2680. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20112680
Zhao X, Fang J, Li S, Gaur U, Xing X, Wang H, Zheng W. Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway. International Journal of Molecular Sciences. 2019; 20(11):2680. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20112680
Chicago/Turabian StyleZhao, Xia, Jiankang Fang, Shuai Li, Uma Gaur, Xingan Xing, Huan Wang, and Wenhua Zheng. 2019. "Artemisinin Attenuated Hydrogen Peroxide (H2O2)-Induced Oxidative Injury in SH-SY5Y and Hippocampal Neurons via the Activation of AMPK Pathway" International Journal of Molecular Sciences 20, no. 11: 2680. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20112680