Role of the G-Protein-Coupled Receptor Signaling Pathway in Insecticide Resistance
Abstract
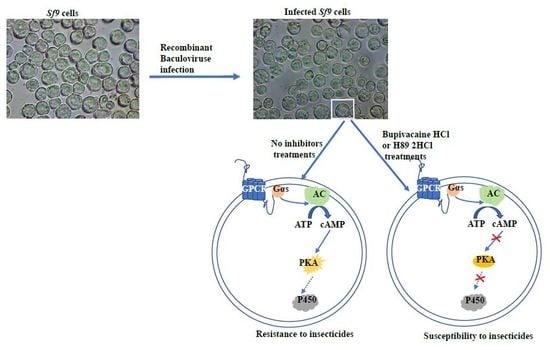
:1. Introduction
2. Results
2.1. Effect of Gene Expression Internalization on cAMP Signaling
2.2. Dynamic Changes of the PKA Activity in Gene Expression Cell Lines
2.3. Regulation Function of the Effectors on P450 Gene Expression in Sf9 Cells
2.4. Effects of cAMP Production Inhibitor on Cell Lines
2.5. Potent Effects of PKA Inhibitor on Cell Lines
2.6. Synergistic Effects of cAMP and PKA Inhibitors on the Toxicity of Permethrin in Culex Mosquito Larva
3. Discussion
4. Materials and Methods
4.1. Gene Construction and Cell Culture
4.2. cAMP Measurement
4.3. Protein Kinase A Activity Assay
4.4. RNA Isolation, cDNA Preparation, and Quantitative Real-Time PCR (qRT-PCR)
4.5. Cell Viability Assay
4.6. Mosquito Strains and Bioassay
4.7. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seifert, R.; Wenzel-Seifert, K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. N-S Arch. Pharmacol. 2002, 366, 381–416. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.A.; IJzerman, A.P. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends. Pharmacol. Sci. 2006, 27, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G. Constitutive activity and inverse agonists of G protein-coupled receptors: a current perspective. Mol. Pharmacol. 2003, 64, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Marinissen, M.J.; Gutkind, J.S. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends. Pharmacol. Sci. 2001, 22, 368–376. [Google Scholar] [CrossRef]
- Kost, T.A.; Condreay, J.P.; Jarvis, D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005, 23, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Akermoun, M.; Koglin, M.; Zvalova-Iooss, D.; Folschweiller, N.; Dowell, S.J.; Gearing, K.L. Characterization of 16 human G protein-coupled receptors expressed in baculovirus-infected insect cells. Protein Expres. Purif. 2005, 44, 65–74. [Google Scholar] [CrossRef]
- Matarazzo, V.; Ronin, C. Human olfactory receptors: recombinant expression in the baculovirus/sf9 insect cell system, functional characterization, and odorant identification. Olfactory Recept. Methods Protoc. 2013, 109–122. [Google Scholar] [CrossRef]
- Caers, J.; Verlinden, H.; Zels, S.; Vandersmissen, H.P.; Vuerinckx, K.; Schoofs, L. More than two decades of research on insect neuropeptide GPCRs: an overview. Front. Endocrinol. 2012, 3, 151. [Google Scholar] [CrossRef] [Green Version]
- Perez, D.M. From plants to man: The GPCR “tree of life”. Mol. Pharmacol. 2005, 67, 1383–1384. [Google Scholar] [CrossRef]
- Audsley, N.; Down, R.E. G protein coupled receptors as targets for next generation pesticides. Insect Biochem. Mol. Biol. 2015, 67, 27–37. [Google Scholar] [CrossRef]
- Li, T.; Cao, C.W.; Yang, T.; Zhang, L.; He, L.; Xi, Z.Y.; Bian, G.W.; Liu, N. A G-protein-coupled receptor regulation pathway in cytochrome P450-mediated permethrin-resistance in mosquitoes, Culex quinquefasciatus. Sci. Rep. 2015, 5, 17772. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Zhang, L.; Liu, N. Role of G-protein-coupled Receptor-related Genes in Insecticide Resistance of the Mosquito, Culex quinquefasciatus. Sci. Rep. 2014, 4, 6474. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, N. Regulation of P450-mediated permethrin resistance in Culex quinquefasciatus by the GPCR/Gαs/AC/cAMP/PKA signaling cascade. Biochem. Biophys. Rep. 2017, 12, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, M.; Hilliou, F.; Fricaux, T.; Audant, P.; Feyereisen, R.; Le Goff, G. Cytochrome P450s from the fall armyworm (Spodoptera frugiperda): responses to plant allelochemicals and pesticides. Insect Mol. Biol. 2015, 24, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef]
- Liu, N.; Li, M.; Gong, Y.H.; Liu, F.; Li, T. Cytochrome P450s-Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, T.; Feng, Y.; Liu, N. The function of two P450s, CYP9M10 and CYP6AA7, in the permethrin resistance of Culex quinquefasciatus. Sci. Rep. 2017, 7, 587. [Google Scholar] [CrossRef]
- Hur, E.M.; Kim, K.T. G protein-coupled receptor signalling and cross-talk—Achieving rapidity and specificity. Cell Signal. 2002, 14, 397–405. [Google Scholar] [CrossRef]
- Motte, E.; Le Stunff, C.; Briet, C.; Dumaz, N.; Silve, C. Modulation of signaling through GPCR-cAMP-PKA pathways by PDE4 depends on stimulus intensity: Possible implications for the pathogenesis of acrodysostosis without hormone resistance. Mol. Cellul. Endocrinol. 2017, 442, 1–11. [Google Scholar] [CrossRef]
- Natarajan, M.; Lin, K.M.; Hsueh, R.C.; Sternweis, P.C.; Ranganathan, R. A global analysis of cross-talk in a mammalian cellular signalling network. Nat. Cell Biol. 2006, 8, 571–580. [Google Scholar] [CrossRef]
- Houston, C.; Wenzel-Seifert, K.; Bürckstümmer, T.; Seifert, R. The human histamine H2-receptor couples more efficiently to Sf9 insect cell Gs-proteins than to insect cell Gq-proteins: limitations of Sf9 cells for the analysis of receptor/Gq-protein coupling. J. Neurochem. 2002, 80, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Pulliainen, A.T.; Pieles, K.; Brand, C.S.; Hauert, B.; Böhm, A.; Quebatte, M.; Wepf, A.; Gstaiger, M.; Aebersold, R.; Dessauer, C.W. Bacterial effector binds host cell adenylyl cyclase to potentiate Gαs-dependent cAMP production. Proc. Natl. Acad. Sci. USA 2012, 109, 9581–9586. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, R.; Leith, J.; Prielipp, R.; Cole, L. Bupivacaine inhibits cyclic-3’,5’-adenosine monophosphate production. A possible contributing factor to cardiovascular toxicity. Anesthesiology 1993, 79, 88–95. [Google Scholar]
- Chijiwa, T.; Mishima, A.; Hagiwara, M.; Sano, M.; Hayashi, K.; Inoue, T.; Naito, K.; Toshioka, T.; Hidaka, H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990, 265, 5267–5272. [Google Scholar] [PubMed]
- Wittwer, C.T.; Herrmann, M.G.; Moss, A.A.; Rasmussen, R.P. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques 1997, 22, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, N. Inheritance of Permethrin Resistance in Culex quinquefasciatus. J. Med. Entomol. 2010, 47, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, H.; Zhang, L.; Liu, N. Kdr allelic variation in pyrethroid resistant mosquitoes, Culex quinquefasciatus (S.). Biochem. Biophys. Res. Commun. 2006, 345, 774–780. [Google Scholar] [CrossRef]






| Primer Description | Primer Name | Primer Sequence |
|---|---|---|
| GPCR020021 | GPCR020021 F | 5′CACCATGGCATCTTACGCAGCATG3′ |
| GPCR020021 R | 5′TTAAGCCTCGATCTTCTCCGC3′ | |
| Gαs006458 | Gαs006458F | 5′CACCATGGGTTGCTTCGGATC3′ |
| Gαs006458R | 5′CTATAACAGCTCGTATTGCCGCA3′ | |
| AC007240 | AC007240 F | 5′CACCATGTCCCTGTTGCG3′ |
| AC007240 R | 5′TTAGTTCTGCTCCTTGGCGATGC3′ | |
| PKA018257 | PKA018257F | 5′CACCATGGGAAACAACGCAACTTCA3′ |
| PKA018257R | 5′TTAAAATTCTGCAAATTCTTTTGC3′ | |
| L-18 | L-18 F | 5′-CGTATCAACCGACCTCCACT-3′ |
| L-18 R | 5′-AGGCACCTTGTAGAGCCTCA-3′ | |
| G6PD | G6PD F | 5′-GGCCCTGTGGCTAACAGAAT-3′ |
| G6PD R | 5′-CATCGTCTCTACCAAAAGGCTTC-3′ | |
| SfCYP9A30 | SfCYP9A30 F | 5′-GTCCTGGTGGCTGTGGTATT-3′ |
| SfCYP9A30 R | 5′-GTGCGAAAAATGATCGTGTG-3′ | |
| SfCYP9A31 | SfCYP9A31 F | 5′-ATGCTCGTCTTGGTCTGGTT-3′ |
| SfCYP9A31 R | 5′-CTGCCCATGTTACCGAAGAT-3′ | |
| SfCYP9A32 | SfCYP9A32 F | 5′-ATCATTCGTAAGGGCCAGTG-3′ |
| SfCYP9A32 R | 5′-AAGTGAACGGGACGATTTTG-3′ | |
| SfCYP333B4 | SfCYP333B4 F | 5′-GAATTATGCCGGTGGTGTCT-3′ |
| SfCYP333B4 R | 5′-TAGCGACATGTCTCGGTGAG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Liu, N. Role of the G-Protein-Coupled Receptor Signaling Pathway in Insecticide Resistance. Int. J. Mol. Sci. 2019, 20, 4300. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174300
Li T, Liu N. Role of the G-Protein-Coupled Receptor Signaling Pathway in Insecticide Resistance. International Journal of Molecular Sciences. 2019; 20(17):4300. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174300
Chicago/Turabian StyleLi, Ting, and Nannan Liu. 2019. "Role of the G-Protein-Coupled Receptor Signaling Pathway in Insecticide Resistance" International Journal of Molecular Sciences 20, no. 17: 4300. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174300