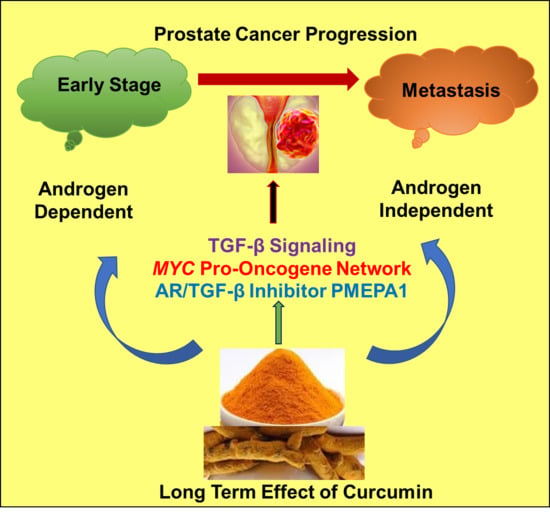
Curcumin-Gene Expression Response in Hormone Dependent and Independent Metastatic Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Gene Expression Responses to Curcumin in Androgen-Dependent LNCaP Cells and Androgen-Independent Metastatic Prostate Cancer C4-2B Cells
2.2. Defining the Prostate Cancer Signature of Differentially Regulated Genes Common in Both LNCaP and C4-2B Cells Post-Curcumin Treatment
2.3. Gene Ontology and Network Analysis of Differentially Expressed Genes Unique to Androgen Responsive Less Aggressive LNCaP Cells
2.4. Gene Ontology and Network Analysis of Differentially Expressed Genes Unique to Androgen-Independent Highly Metastatic C4-2B Cells
2.5. Canonical Pathway Analysis of Curcumin Response Genes in Both LNCaP and C4-2B Cells
2.6. Comparison of Differentially Regulated Genes in LNCaP and C4-2B at 48 h
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. RNA Extraction, Labeling and Gene Expression Analysis
4.3. Affymterix Gene Chip Microarray Data Analysis
4.4. Gene ontology (GO) and Pathway Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
DoD Disclaimer
Abbreviations
| TGF-β | Transforming growth factor beta |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PTEN | Phosphatase and tensin homolog |
| MAPK | Mitogen-activated protein kinase |
| EGFR | Epidermal growth factor receptor |
| AR | Androgen Receptor |
| TNF | Tumor necrosis factor |
| COX-2 | Cyclooxygenase-2 |
| 5-LOX | 5-lipoxygenase |
| TNF | Tumor necrosis factor |
| IL6 | Interleukin 6 |
| KLK3 | Kallikrein related peptidase 3 |
| PSA | Prostate-specific antigen |
| TMPRSS2 | Transmembrane serine protease 2 |
| NKX3.1 | NK3 Homeobox 1 |
| PMEPA1 | Prostate transmembrane protein, androgen induced 1 |
| HMOX1 | Heme Oxygenase-1 |
| ATF–3 | Cyclic AMP-dependent transcription factor |
| GePS | Genomatix Pathway System |
| RAF1 | Raf-1 Proto-Oncogene, Serine/Threonine Kinase |
| BCL6 | B-Cell CLL/Lymphoma 6 |
| IGF1R | Insulin-Like Growth Factor 1 Receptor |
| SMAD7 | SMAD Family Member 7 |
| FOXO3 | Forkhead Box O3 |
| AKT1 | V-Akt Murine Thymoma Viral Oncogene Homolog 1 |
| RAD51 | RAD51 Recombinase |
| SOX4 | (SRY (Sex Determining Region Y)-Box 4 |
| WT1 | Wilms Tumor 1 |
| E2F2 | E2F Transcription Factor 2 |
| MALAT1 | Metastasis Associated Lung Adenocarcinoma Transcript 1 |
| FOXM1 | Forkhead box M1 |
| EWSR1 | Ewing sarcoma breakpoint region 1 |
| SESN2 | Sestrin-2 |
| GCLM | Glutamate-Cysteine Ligase Modifier Subunit |
| AFF4 | AF4/FMR2 Family Member 4 |
| ELF3 | E74 Like ETS Transcription Factor 3 |
| CREM | CAMP Responsive Element Modulator |
| BIRC4 | Bculoviral IAP repeat-containing protein 4 |
| RIOK3 | RIO Kinase 3 |
| PIK3R3 | Phosphoinositide-3-Kinase Regulatory Subunit 3 |
| UBE2H | Ubiquitin Conjugating Enzyme E2 H |
| C6orf62 | Chromosome 6 open reading frame 62 |
| DDX11 | DEAD/H-Box Helicase 11 |
| CROT | Carnitine O-Octanoyltransferase |
| HIG2 | Hypoxia-inducible gene 2 |
| C1orf116 | Chromosome 1 Open Reading Frame 116 |
| DFFB | DNA Fragmentation Factor Subunit Beta |
| GPC6 | Glypican-6 |
| C15orf20 | Chromosome 15 Open Reading Frame 20 |
| ADRB2 | Adrenoceptor Beta 2 |
| SLC16A6 | Solute Carrier Family 16 Member 6 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Biemar, F.; Foti, M. Global progress against cancer-challenges and opportunities. Cancer Biol. Med. 2013, 10, 183–186. [Google Scholar] [PubMed]
- Prostate Cancer: Risk Factors and Prevention. Available online: https://www.cancer.net/cancer-types/prostate-cancer/risk-factors-and-prevention (accessed on 2 October 2019).
- Prostate Cancer Risk Factors. Available online: https://www.pcf.org/patient-resources/family-cancer-risk/prostate-cancer-risk-factors/ (accessed on 2 October 2019).
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharm. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Pugazhenthi, S.; Nesterova, A.; Sable, C.; Heidenreich, K.A.; Boxer, L.M.; Heasley, L.E.; Reusch, J.E. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J. Biol. Chem. 2000, 275, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Yu, S.; Shen, G.; Khor, T.O.; Kim, J.H.; Kong, A.N. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2609–2620. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Ahn, K.S.; Bae, E.; Jeon, S.S.; Choi, H.Y. The effects of curcumin on the invasiveness of prostate cancer in vitro and in vivo. Prostate Cancer Prostatic Dis. 2006, 9, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Nonn, L.; Duong, D.; Peehl, D.M. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis 2006, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.O.; Keum, Y.S.; Lin, W.; Kim, J.H.; Hu, R.; Shen, G.; Xu, C.; Gopalakrishnan, A.; Reddy, B.; Zheng, X.; et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC–3 prostate xenografts in immunodeficient mice. Cancer Res. 2006, 66, 613–621. [Google Scholar] [CrossRef]
- Dehm, S.M.; Tindall, D.J. Molecular regulation of androgen action in prostate cancer. J. Cell Biochem. 2006, 99, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Steineck, G.; Kelly, W.K. Hormone-refractory [D3] prostate cancer: Refining the concept. Urology 1995, 46, 142–148. [Google Scholar] [CrossRef]
- Richter, E.; Srivastava, S.; Dobi, A. Androgen Receptor and prostate cancer. Prostate Cancer Prostatic Dis. 2007, 10, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yasunaga, Y.; Segawa, T.; Ko, D.; Moul, J.W.; Srivastava, S.; Rhim, J.S. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int. J. Oncol. 2002, 21, 825–830. [Google Scholar] [CrossRef]
- Ohtsu, H.; Xiao, Z.; Ishida, J.; Nagai, M.; Wang, H.K.; Itokawa, H.; Su, C.Y.; Shih, C.; Chiang, T.; Chang, E.; et al. Antitumor agents 217. Curcumin analogues as novel androgen receptor antagonists with potential as anti-prostate cancer agents. J. Med. Chem. 2002, 45, 5037–5042. [Google Scholar] [CrossRef]
- Huang, M.T.; Lou, Y.R.; Ma, W.; Newmark, H.L.; Reuhl, K.R.; Conney, A.H. Inhibitory effects of dietary curcumin on forestomach, duodenal and colon carcinogenesis in mice. Cancer Res. 1994, 54, 5841–5847. [Google Scholar]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J. Mol. Med. 2007, 19, 165–172. [Google Scholar] [CrossRef]
- Yan, C.; Jamaluddin, M.S.; Aggarwal, B.; Myers, J.; Boyd, D.D. Gene expression profiling identifies activating transcription factor 3 as a novel contributor to the proapoptotic effect of curcumin. Mol. Cancer. 2005, 4, 233–241. [Google Scholar]
- Zhang, H.N.; Yu, C.X.; Zhang, P.J.; Chen, W.W.; Jiang, A.L.; Kong, F.; Deng, J.T.; Zhang, J.Y.; Young, C.Y. Curcumin down-regulates homeobox gene NKX3.1 in prostate cancer cell LNCaP. Acta. Pharmacol. Sin. 2007, 28, 423–430. [Google Scholar] [CrossRef]
- Hasima, N.; Aggarwal, B.B. Cancer-linked targets modulated by curcumin. Int. J. Biochem Mol. Biol. 2012, 3, 328–351. [Google Scholar]
- Thangapazham, R.L.; Shaheduzzaman, S.; Kim, K.H.; Passi, N.; Tadese, A.; Vahey, M.; Dobi, A.; Srivastava, S.; Maheshwari, R.K. Androgen responsive and refractory prostate cancer cells exhibit distinct curcumin regulated transcriptome. Cancer Biol. Ther. 2008, 7, 1427–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barfeld, S.J.; Urbanucci, A.; Itkonen, H.M.; Fazli, L.; Hicks, J.L.; Thiede, B.; Rennie, P.S.; Yegnasubramanian, S.; DeMarzo, A.M.; Mills, I.G. c-Myc Antagonises the Transcriptional Activity of the Androgen Receptor in Prostate Cancer Affecting Key Gene Networks. EBioMedicine 2017, 18, 83–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teiten, M.H.; Gaascht, F.; Cronauer, M.; Henry, E.; Dicato, M.; Diederich, M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int. J. Oncol. 2011, 38, 603–611. [Google Scholar] [PubMed] [Green Version]
- Choi, H.Y.; Lim, J.E.; Hong, J.H. Curcumin interrupts the interaction between the androgen receptor and Wnt/β-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010, 13, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.; Yang, X.; Chen, M.W.; Vacherot, F.; Buttyan, R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J. Cell Biochem. 2006, 99, 402–410. [Google Scholar] [CrossRef]
- Singh, R.P.; Agarwal, R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr. Relat. Cancer. 2006, 13, 751–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, K.T.; Figg, W.D. The potential role of curcumin in prostate cancer: The importance of optimizing pharmacokinetics in clinical studies. Transl. Cancer Res. 2016, 5 (Suppl. 6), S1107–S1110. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, C. Emerging roles of ATF3 in the suppression of prostate cancer. Mol. Cell Oncol. 2015, 3, e1010948. [Google Scholar] [CrossRef] [Green Version]
- Nna, E.; Madukwe, J.; Egbujo, E.; Obiorah, C.; Okolie, C.; Echejoh, G.; Yahaya, A.; Adisa, J.; Uzoma, I. Gene expression of Aurora kinases in prostate cancer and nodular hyperplasia tissues. Med. Princ. Pract. 2013, 22, 138–143. [Google Scholar] [CrossRef]
- Addepalli, M.K.; Ray, K.B.; Kumar, B.; Ramnath, R.L.; Chile, S.; Rao, H. RNAi-mediated knockdown of AURKB and EGFR shows enhanced therapeutic efficacy in prostate tumor regression. Gene Ther. 2010, 17, 52–59. [Google Scholar] [CrossRef]
- Fournier, P.G.; Juárez, P.; Jiang, G.; Clines, G.A.; Niewolna, M.; Kim, H.S.; Walton, H.W.; Peng, X.H.; Liu, Y.; Mohammad, K.S.; et al. The TGF-β Signaling Regulator PMEPA1 Suppresses Prostate Cancer Metastases to Bone. Cancer Cell 2015, 27, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mohamed, A.A.; Sharad, S.; Umeda, E.; Song, Y.; Young, D.; Petrovics, G.; McLeod, D.G.; Sesterhenn, I.A.; Sreenath, T.; et al. Silencing of PMEPA1 accelerates the growth of prostate cancer cells through AR, NEDD4 and PTEN. Oncotarget 2015, 6, 15137–15149. [Google Scholar] [CrossRef] [PubMed]
- Podkalicka, P.; Mucha, O.; Józkowicz, A.; Dulak, J.; Łoboda, A. Heme oxygenase inhibition in cancers: Possible tools and targets. Contemp. Oncol. 2018, 22, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, Z.; Li, M.; Csizmadia, E.; Döme, B.; Johansson, M.; Persson, J.L.; Seth, P.; Otterbein, L.; Wegiel, B. Heme oxygenase-1 in macrophages controls prostate cancer progression. Oncotarget 2015, 6, 33675–33688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paez, A.V.; Pallavicini, C.; Schuster, F.; Valacco, M.P.; Giudice, J.; Ortiz, E.G.; Anselmino, N.; Labanca, E.; Binaghi, M.; Salierno, M.; et al. Heme oxygenase-1 in the forefront of a multi-molecular network that governs cell-cell contacts and filopodia-induced zippering in prostate cancer. Cell Death Dis. 2016, 7, e2570. [Google Scholar] [CrossRef] [PubMed]
- Gueron, G.; Giudice, J.; Valacco, P.; Paez, A.; Elguero, B.; Toscani, M.; Jaworski, F.; Leskow, F.C.; Cotignola, J.; Marti, M.; et al. Heme-oxygenase-1 implications in cell morphology and the adhesive behavior of prostate cancer cells. Oncotarget 2014, 5, 4087–4102. [Google Scholar] [CrossRef]
- Thacker, P.C.; Karunagaran, D. Curcumin and emodin down-regulate TGF-β signaling pathway in human cervical cancer cells. PLoS ONE 2015, 10, e0120045. [Google Scholar] [CrossRef]
- Ramamoorthi, G.; Sivalingam, N. Molecular mechanism of TGF-β signaling pathway in colon carcinogenesis and status of curcumin as chemopreventive strategy. Tumour Biol. 2014, 35, 7295–7305. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, A. Disruption of transforming growth factor-beta signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-gamma in rat hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G113–G123. [Google Scholar] [CrossRef]
- Chan, J.J.; Kwok, Z.H.; Chew, X.H.; Zhang, B.; Liu, C.; Soong, T.W.; Yang, H.; Tay, Y. A FTH1 gene:pseudogene:microRNA network regulates tumorigenesis in prostate cancer. Nucleic Acids Res. 2018, 46, 1998–2011. [Google Scholar] [CrossRef]
- Zeng, M.; Li, F.; Wang, L.; Chen, C.; Huang, X.; Wu, X.; She, W.; Zhou, L.; Tao, Z. Down-regulated cytoplasmic polyadenylation element-binding protein-4 is associated with the carcinogenesis of head and neck squamous cell carcinoma. Oncol. Lett. 2018, 15, 3226–3232. [Google Scholar]
- Lee, K.Y.; Im, J.S.; Shibata, E.; Park, J.; Handa, N.; Kowalczykowski, S.C.; Dutta, A. MCM8–9 complex promotes resection of double-strand break ends by MRE11-RAD50-NBS1 complex. Nat. Commun. 2015, 6, 7744. [Google Scholar] [CrossRef] [PubMed]
- Sharad, S.; Srivastava, A.; Ravulapalli, S.; Parker, P.; Chen, Y.; Li, H.; Petrovics, G.; Dobi, A. Prostate cancer gene expression signature of patients with high body mass index. Prostate Cancer Prostatic Dis. 2011, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Shishodia, S. Molecular mechanisms of curcumin action: Gene expression. Biofactors 2013, 39, 37–55. [Google Scholar] [CrossRef] [PubMed]






| LNCaP (>2 Fold Change) | |||||
| 3 h | 6 h | 12 h | 24 h | 48 h | |
| Up-regulated | 704 | 822 | 1273 | 806 | 41 |
| Down-regulated | 621 | 1422 | 1682 | 934 | 119 |
| C4-2B (>2 Fold Change) | |||||
| 3 h | 6 h | 12 h | 24 h | 48 h | |
| Up-regulated | 644 | 977 | 1119 | 410 | 105 |
| Down-regulated | 439 | 508 | 943 | 318 | 343 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katta, S.; Srivastava, A.; Thangapazham, R.L.; Rosner, I.L.; Cullen, J.; Li, H.; Sharad, S. Curcumin-Gene Expression Response in Hormone Dependent and Independent Metastatic Prostate Cancer Cells. Int. J. Mol. Sci. 2019, 20, 4891. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194891
Katta S, Srivastava A, Thangapazham RL, Rosner IL, Cullen J, Li H, Sharad S. Curcumin-Gene Expression Response in Hormone Dependent and Independent Metastatic Prostate Cancer Cells. International Journal of Molecular Sciences. 2019; 20(19):4891. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194891
Chicago/Turabian StyleKatta, Shilpa, Arun Srivastava, Rajesh L. Thangapazham, Inger L. Rosner, Jennifer Cullen, Hua Li, and Shashwat Sharad. 2019. "Curcumin-Gene Expression Response in Hormone Dependent and Independent Metastatic Prostate Cancer Cells" International Journal of Molecular Sciences 20, no. 19: 4891. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194891