Neutrophil Maturation and Survival Is Controlled by IFN-Dependent Regulation of NAMPT Signaling
Abstract
:1. Introduction
2. Results
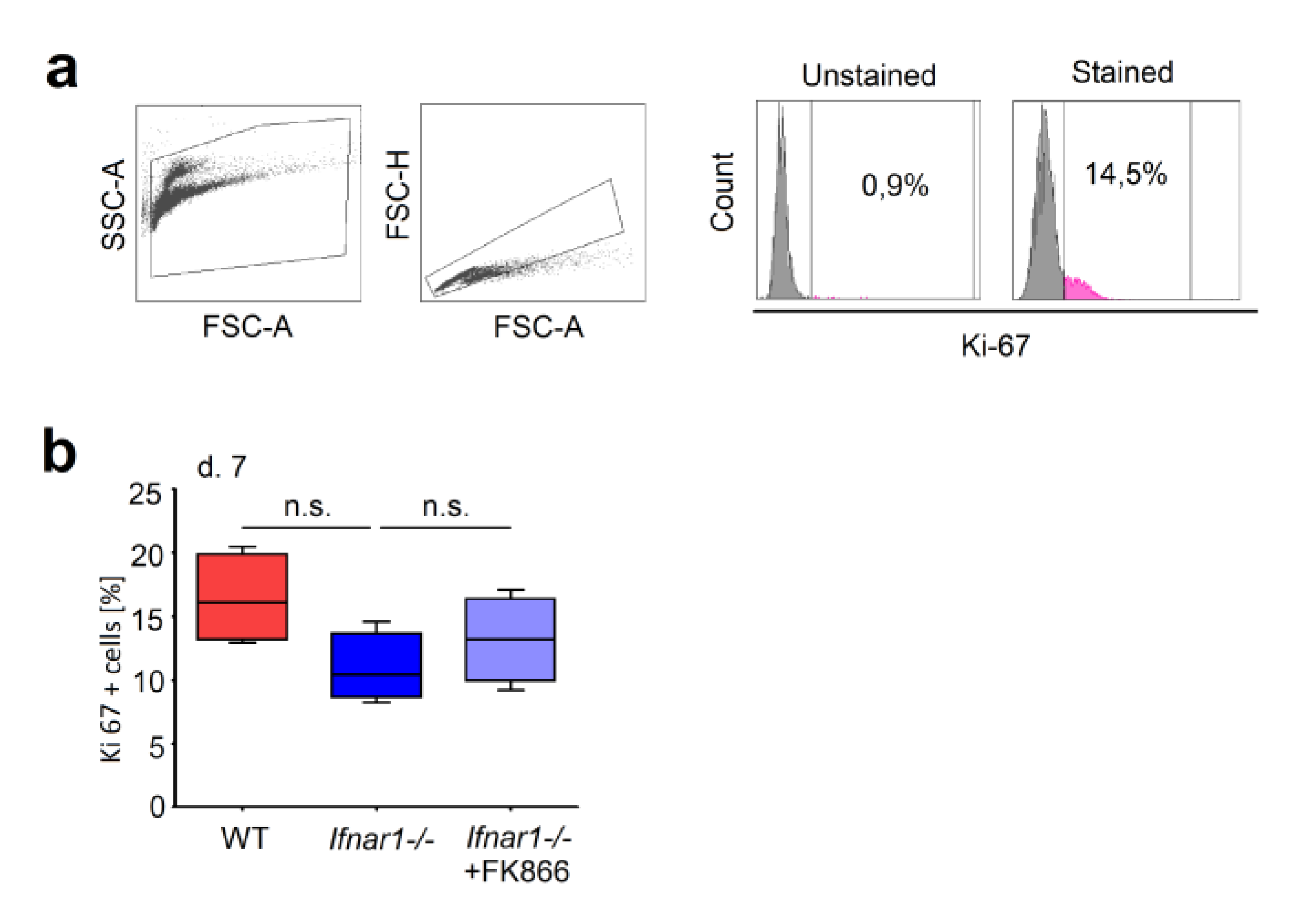
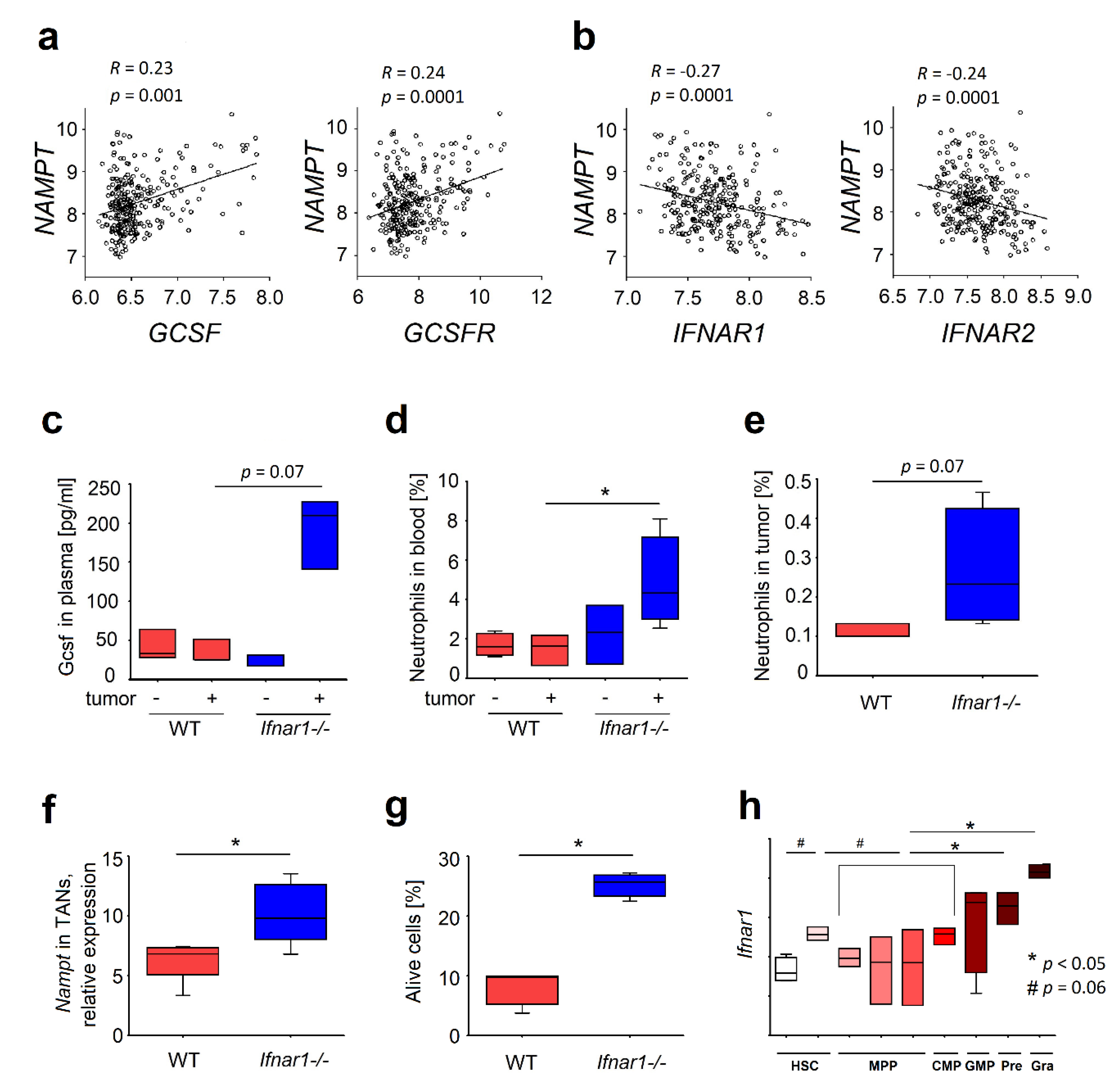
2.1. Elevated Expression of NAMPT in IFN-Deficient Mice Is Associated with Elevated Survival of Neutrophils
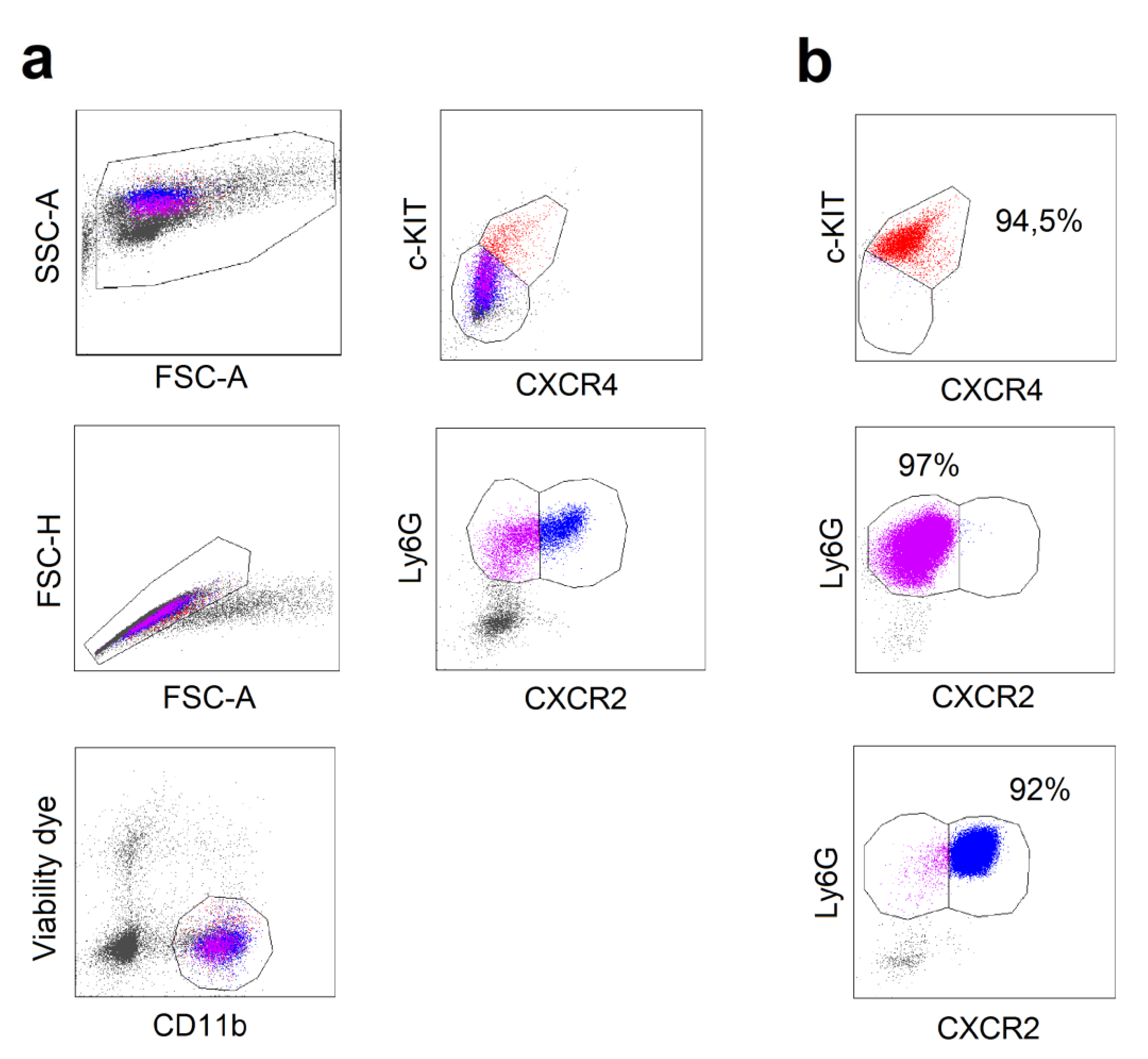
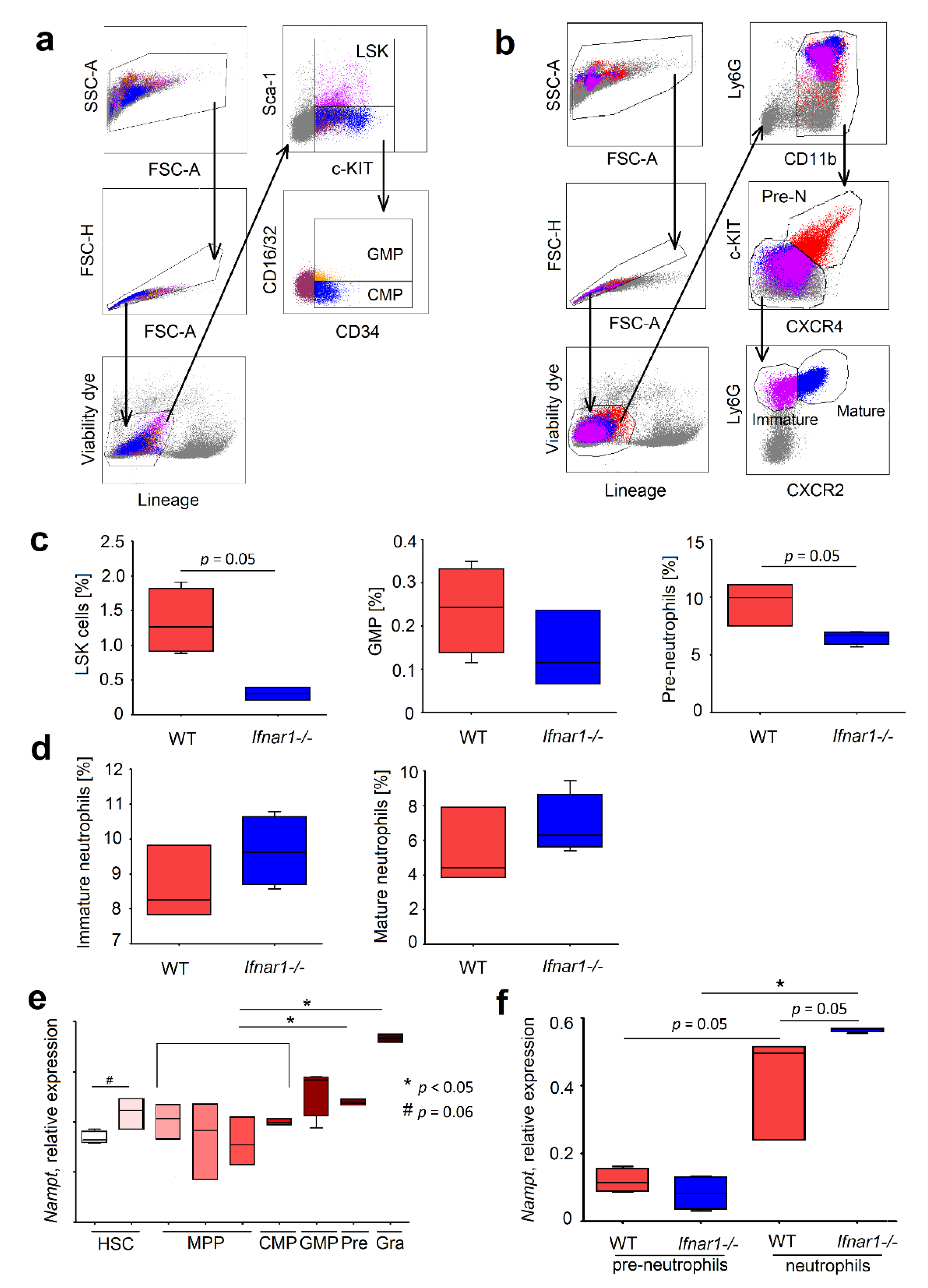
2.2. Different Composition of Bone Marrow Neutrophil Progenitors in Ifnar1-/- Mice Is NAMPT-Dependent and Associated with Altered Apoptosis
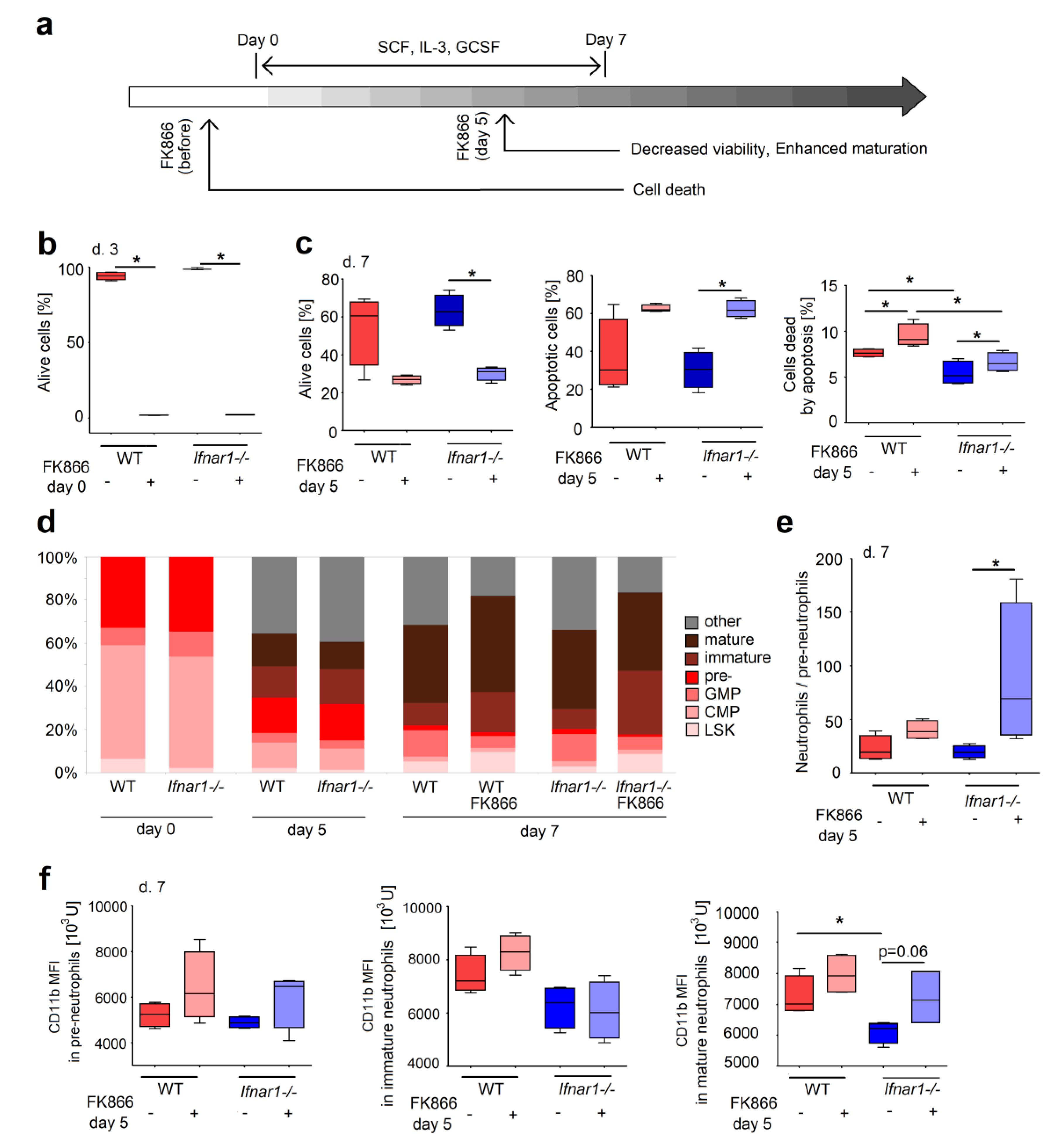
2.3. Direct NAMPT Inhibition in Isolated BM Progenitors Regulates Their Survival and Differentiation, Depending on the Maturation Stage
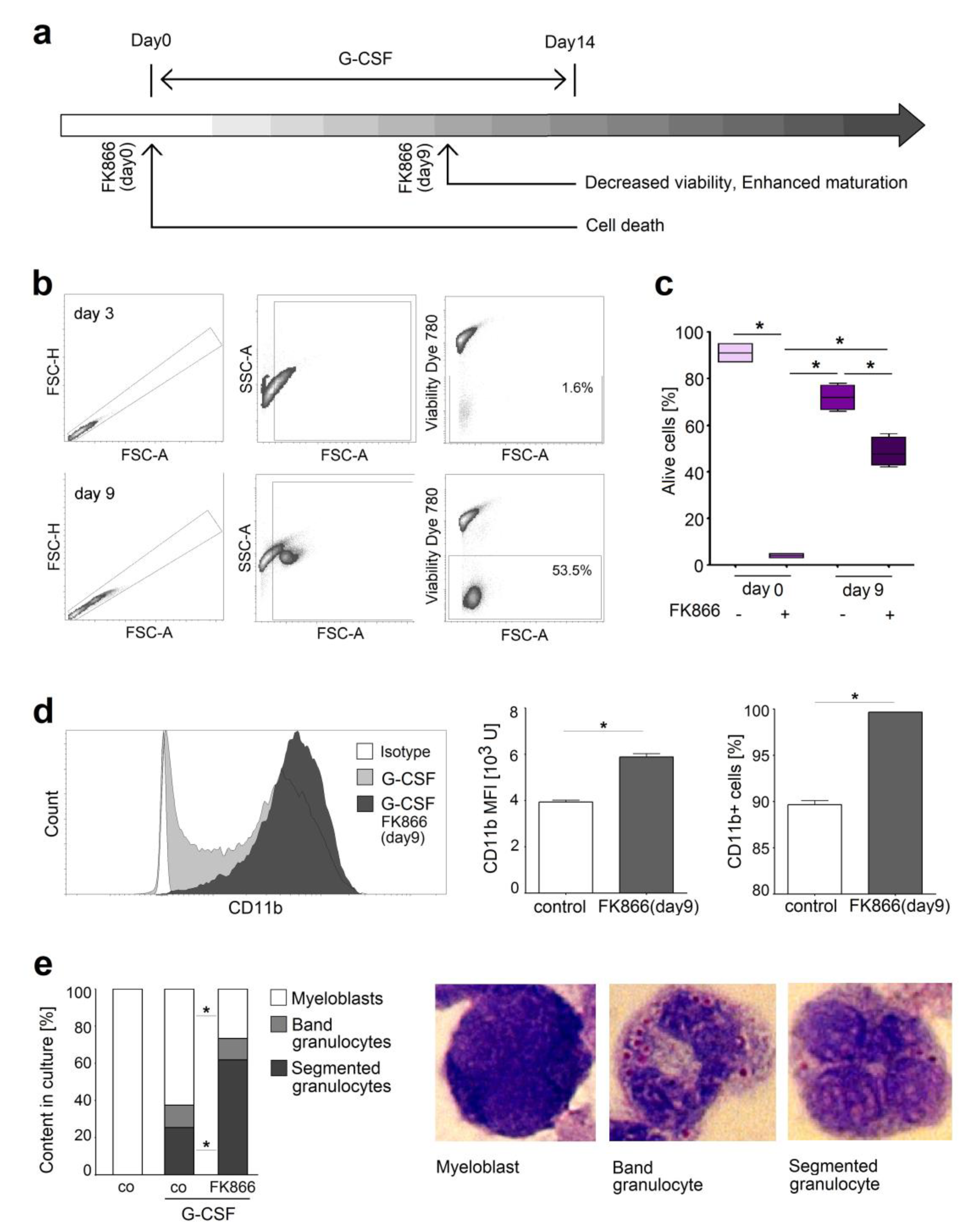
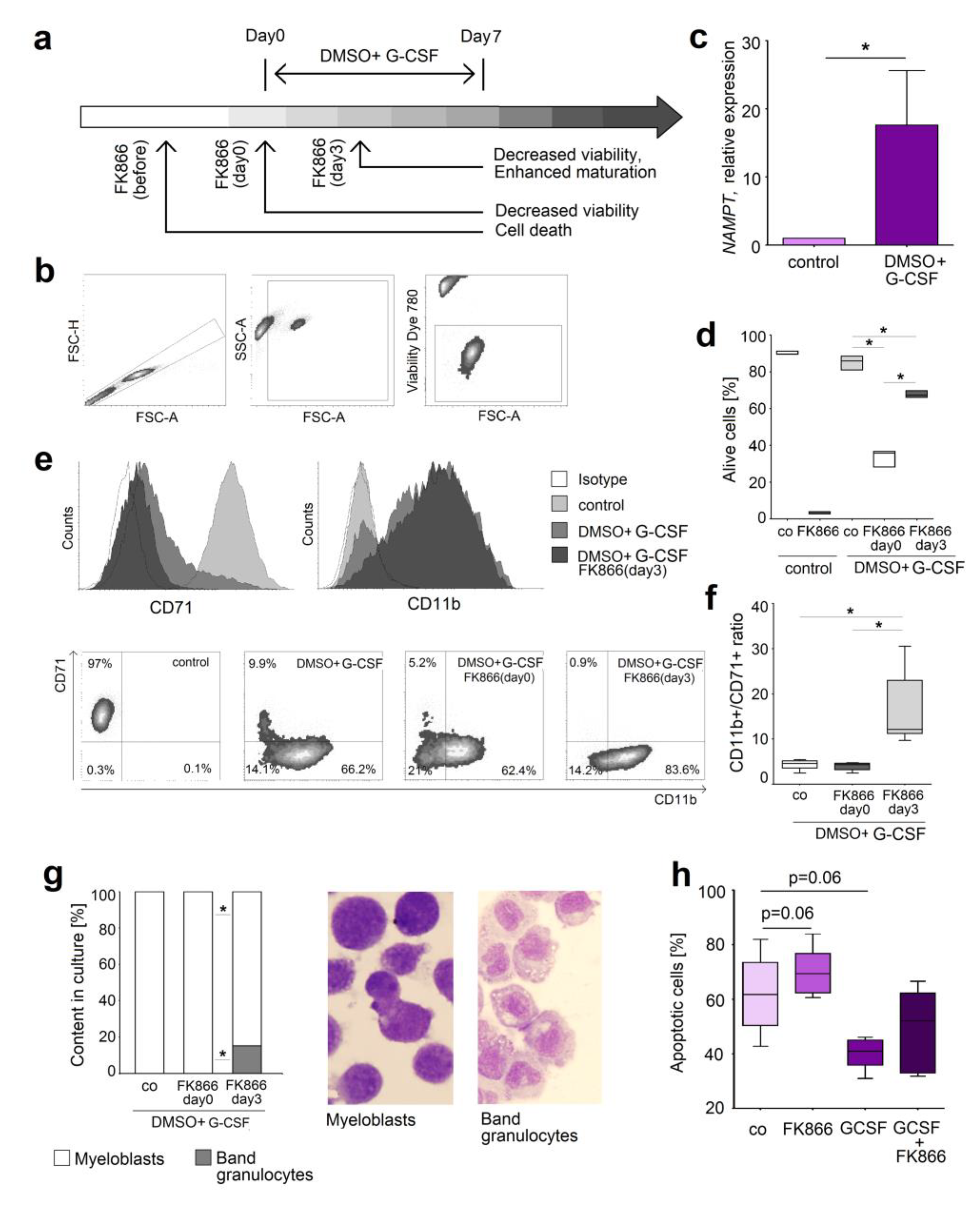
2.4. NAMPT Is Responsible for Survival and Delayed Differentiation of Murine Myeloblast Cell Line
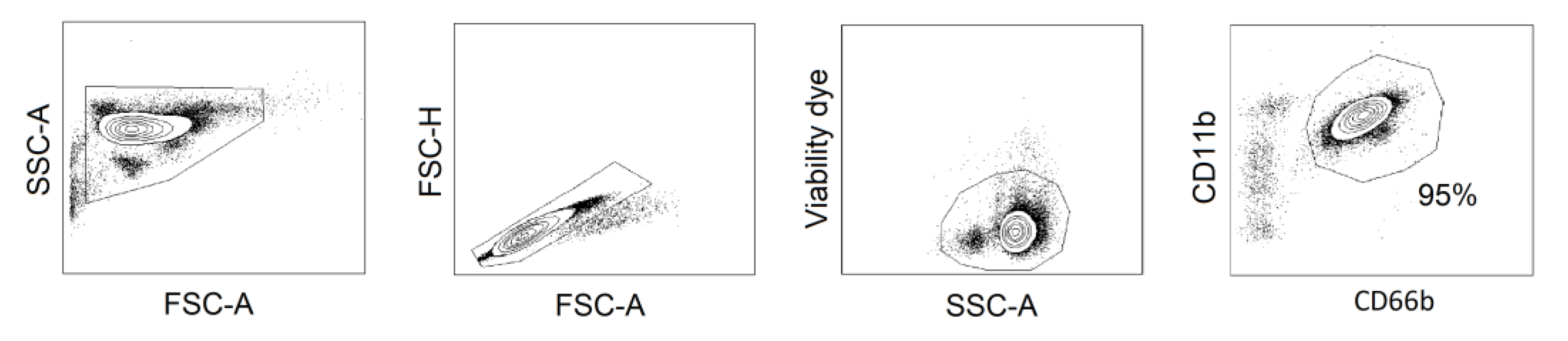
2.5. Inhibition of NAMPT in Human Neutrophil Progenitors Reduces Their Survival and Improved Differentiation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tumor Model and Tumor-Associated Neutrophils
4.3. Bone Marrow Cells Isolation
4.4. Murine Neutrophil Isolation
4.5. In Vitro Maturation of Isolated Murine Bone Marrow Progenitors
4.6. In Vitro Proliferation of Isolated Murine Bone Marrow Progenitors
4.7. Cell Lines
4.8. Morphological Analysis
4.9. Isolation and Treatment of Human Peripheral Blood Neutrophils
4.10. Apoptosis Assay
4.11. Flow Cytometry
4.12. Antibodies
4.13. Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
4.14. G-CSF Measurements
4.15. Analysis of the Data Deposited in the Gene Expression Omnibus Databases
4.16. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| G-CSF | Granulocyte-colony stimulating factor |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| IFN | Interferon |
| IFNAR | Interferon receptor |
| HSC | Hematopoietic stem cells |
| CMP | Common myeloid progenitor |
| GMP | Granulocyte–monocyte progenitor |
| NAD | Nicotinamide adenine dinucleotide |
| mTOR | Mammalian target of rapamycin |
| 7AAD | 7-Aminoactinomycin |
Appendix A
Appendix B
References
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A Clonogenic Common Myeloid Progenitor That Gives Rise to All Myeloid Lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Evrard, M.; Kwok, I.W.H.; Chong, S.Z.; Teng, K.W.W.; Becht, E.; Chen, J.; Sieow, J.L.; Penny, H.L.; Ching, G.C.; Devi, S.; et al. Developmental Analysis of Bone Marrow Neutrophils Reveals Populations Specialized in Expansion, Trafficking, and Effector Functions. Immunity 2018, 48, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Skokowa, J.; Lan, D.; Thakur, B.K.; Wang, F.; Gupta, K.; Cario, G.; Brechlin, A.M.; Schambach, A.; Hinrichsen, L.; Meyer, G.; et al. NAMPT Is Essential for the G-CSF-Induced Myeloid Differentiation Via a NAD(+)-Sirtuin-1-Dependent Pathway. Nat. Med. 2009, 15, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Rongvaux, A.; Shea, R.J.; Mulks, M.H.; Gigot, D.; Urbain, J.; Leo, O.; Andris, F. Pre-B-Cell Colony-Enhancing Factor, Whose Expression Is Up-Regulated in Activated Lymphocytes, Is a Nicotinamide Phosphoribosyltransferase, a Cytosolic Enzyme Involved in NAD Biosynthesis. Eur. J. Immunol. 2002, 32, 3225–3234. [Google Scholar] [CrossRef]
- Chalkiadaki, A.; Guarente, L. The Multifaceted Functions of Sirtuins in Cancer. Nat. Rev. Cancer 2015, 15, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Dorweiler, B.; Cui, D.; Wang, T.; Woo, C.W.; Brunkan, C.S.; Wolberger, C.; Imai, S.; Tabas, I. Extracellular Nampt Promotes Macrophage Survival Via a Nonenzymatic Interleukin-6/STAT3 Signaling Mechanism. J. Biol. Chem. 2008, 283, 34833–34843. [Google Scholar] [CrossRef]
- Pylaeva, E.; Harati, M.D.; Spyra, I.; Bordbari, S.; Strachan, S.; Thakur, B.K.; Hoing, B.; Franklin, C.; Skokowa, J.; Welte, K.; et al. NAMPT Signaling Is Critical for the Proangiogenic Activity of Tumor-Associated Neutrophils. Int. J. Cancer 2019, 144, 136–149. [Google Scholar] [CrossRef]
- Wichmann, G.; Rosolowski, M.; Krohn, K.; Kreuz, M.; Boehm, A.; Reiche, A.; Scharrer, U.; Halama, D.; Bertolini, J.; Bauer, U.; et al. The Role of HPV RNA Transcription, Immune Response-Related Gene Expression and Disruptive TP53 Mutations in Diagnostic and Prognostic Profiling of Head and Neck Cancer. Int. J. Cancer 2015, 137, 2846–2857. [Google Scholar] [CrossRef]
- Granot, Z.; Jablonska, J. Distinct Functions of Neutrophil in Cancer and Its Regulation. Mediators. Inflamm. 2015, 2015, 701067. [Google Scholar] [CrossRef]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils Responsive to Endogenous IFN-Beta Regulate Tumor Angiogenesis and Growth in a Mouse Tumor Model. J. Clin. Investig. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: “N1” Versus “N2” TAN. J. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs Induce Anti-Tumor Polarization of Tumor Associated Neutrophils in Mice and Human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef] [PubMed]
- Pietras, E.M.; Reynaud, D.; Kang, Y.-A.; Carlin, D.; Calero-Nieto, F.J.; Leavitt, A.D.; Stuart, J.M.; Göttgens, B.; Passegué, E. Functionally Distinct Subsets of Lineage-Biased Multipotent Progenitors Control Blood Production in Normal and Regenerative Conditions. Cell Stem Cell 2015, 17, 35–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrone-Smith, T.; Izban, K.F.; Ergin, M.; Cosar, E.F.; Hsi, E.D.; Alkan, S. Transfection of caspase-3 in the caspase-3-deficient Hodgkin’s disease cell line, KMH2, results in enhanced sensitivity to CD95-, TRAIL-, and ARA-C-induced apoptosis. Exp. Hematol. 2001, 29, 572–581. [Google Scholar] [CrossRef]
- Gupta, D.; Shah, H.P.; Malu, K.; Berliner, N.; Gaines, P. Differentiation and characterization of myeloid cells. Curr. Protoc. Immunol. 2014, 104, 22F.5.1–22F.5.28. [Google Scholar]
- Guchhait, P.; Tosi, M.F.; Smith, C.W.; Chakaraborty, A. The murine myeloid cell line 32Dcl3 as a model system for studying neutrophil functions. J. Immunol. Methods 2003, 283, 195–204. [Google Scholar] [CrossRef]
- Bararia, D.; Kwok, H.S.; Welner, R.S.; Numata, A.; Sarosi, M.B.; Yang, H.; Wee, S.; Tschuri, S.; Ray, D.; Weigert, O.; et al. Acetylation of C/EBPalpha Inhibits Its Granulopoietic Function. Nat. Commun. 2016, 7, 10968. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y. Apoptosis of Human Leukemic HL-60 Cells Induced to Differentiate by Treatment With RA or DMSO[Ast]. Cell Res. 1995, 5, 181–186. [Google Scholar] [CrossRef]
- Breitman, T.R.; Selonick, S.E.; Collins, S.J. Induction of Differentiation of the Human Promyelocytic Leukemia Cell Line (HL-60) by Retinoic Acid. Proc. Natl. Acad Sci. USA 1980, 77, 2936–2940. [Google Scholar] [CrossRef]
- Andzinski, L.; Wu, C.F.; Lienenklaus, S.; Kroger, A.; Weiss, S.; Jablonska, J. Delayed Apoptosis of Tumor Associated Neutrophils in the Absence of Endogenous IFN-Beta. Int. J. Cancer 2015, 136, 572–583. [Google Scholar]
- Kim, H.K.; De La Luz, S.M.; Williams, C.K.; Gulino, A.V.; Tosato, G. G-CSF Down-Regulation of CXCR4 Expression Identified As a Mechanism for Mobilization of Myeloid Cells. Blood 2006, 108, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.; De Filippo, K.; Hasenberg, M.; Brandt, C.V.D.; Nye, E.; Hosking, M.P.; Lane, T.E.; Männ, L.; Ransohoff, R.M.; Hauser, A.E.; et al. G-CSF–mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 2011, 117, 4349–4357. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-F.; Andzinski, L.; Kasnitz, N.; Kröger, A.; Klawonn, F.; Lienenklaus, S.; Weiss, S.; Jablonska, J. The lack of type I interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int. J. Cancer 2015, 137, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Essers, M.A.; Offner, S.; Blanco-Bose, W.E.; Waibler, Z.; Kalinke, U.; Duchosal, M.A.; Trumpp, A. IFNalpha Activates Dormant Haematopoietic Stem Cells in Vivo. J. Nature 2009, 458, 904–908. [Google Scholar] [CrossRef]
- Smith, J.N.; Kanwar, V.S.; MacNamara, K.C. Hematopoietic Stem Cell Regulation by Type I and II Interferons in the Pathogenesis of Acquired Aplastic Anemia. Front. Immunol. 2016, 7, 330. [Google Scholar] [CrossRef]
- Buechler, M.B.; Akilesh, H.M.; Hamerman, J.A. Cutting Edge: Direct Sensing of TLR7 Ligands and Type I IFN by the Common Myeloid Progenitor Promotes MTOR/PI3K-Dependent Emergency Myelopoiesis. J. Immunol. 2016, 197, 2577–2582. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, S.-R.; Kim, S.S.; Wee, H.-J.; Bae, M.-K.; Ryu, M.H.; Bae, S.-K. Visfatin promotes cell and tumor growth by upregulating Notch1 in breast cancer. Oncotarget. 2014, 5, 5087–5099. [Google Scholar] [CrossRef] [Green Version]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de, G.T.; Kiess, W. Physiological and Pathophysiological Roles of NAMPT and NAD Metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef]
- Samal, B.; Sun, Y.; Stearns, G.; Xie, C.; Suggs, S.; Mcniece, I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Boil. 1994, 14, 1431–1437. [Google Scholar] [CrossRef]
- Hasmann, M.; Schemainda, I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003, 63, 7436–7442. [Google Scholar]
- Mouchiroud, L.; Houtkooper, R.H.; Auwerx, J. NAD+ metabolism: A therapeutic target for age-related metabolic disease. Crit. Rev. Biochem. Mol. Boil. 2013, 48, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, A.; Dolle, C.; Felici, R.; Ziegler, M. The NAD Metabolome--a Key Determinant of Cancer Cell Biology. Nat. Rev. Cancer 2012, 12, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, T.; Baur, J.A.; Perez, E.; Matsui, T.; Carmona, J.J.; Lamming, D.W.; Souza-Pinto, N.C.; Bohr, V.A.; Rosenzweig, A.; et al. Nutrient-Sensitive Mitochondrial NAD+ Levels Dictate Cell Survival. Cell 2007, 130, 1095–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.G.; Kim, E.O.; Jeong, B.R.; Min, Y.J.; Park, J.W.; Kim, E.S.; Namgoong, I.S.; Kim, Y.I.; Lee, B.J. Visfatin Stimulates Proliferation of MCF-7 Human Breast Cancer Cells. Mol. Cells 2010, 30, 341–345. [Google Scholar] [CrossRef]
- Audrito, V.; Serra, S.; Brusa, D.; Mazzola, F.; Arruga, F.; Vaisitti, T.; Coscia, M.; Maffei, R.; Rossi, D.; Wang, T.; et al. Extracellular Nicotinamide Phosphoribosyltransferase (NAMPT) Promotes M2 Macrophage Polarization in Chronic Lymphocytic Leukemia. Blood 2015, 125, 111–123. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Qiao, S.-B.; Guan, H.-S.; Liu, S.-W.; Meng, X.-M. Effects of Visfatin on Proliferation and Collagen Synthesis in Rat Cardiac Fibroblasts. Horm. Metab. Res. 2010, 42, 507–513. [Google Scholar] [CrossRef]
- Moschen, A.R.; Geiger, S.; Gerner, R.; Tilg, H. Pre-B Cell Colony Enhancing Factor/NAMPT/Visfatin and Its Role in Inflammation-Related Bone Disease. Mutat. Res. 2010, 690, 95–101. [Google Scholar] [CrossRef]
- van der Veer, E.; Ho, C.; O’Neil, C.; Barbosa, N.; Scott, R.; Cregan, S.P.; Pickering, J.G. Extension of Human Cell Lifespan by Nicotinamide Phosphoribosyltransferase. J. Biol. Chem. 2007, 282, 10841–10845. [Google Scholar] [Green Version]
- Kolthur-Seetharam, U.; Dantzer, F.; McBurney, M.W.; de, M.G.; Sassone-Corsi, P. Control of AIF-mediated Cell Death by the Functional Interplay of SIRT1 and PARP-1 in Response to DNA Damage. Cell Cycle 2006, 5, 873–877. [Google Scholar] [CrossRef] [Green Version]
- Thakur, B.K.; Dittrich, T.; Chandra, P.; Becker, A.; Kuehnau, W.; Klusmann, J.H.; Reinhardt, D.; Welte, K. Involvement of P53 in the Cytotoxic Activity of the NAMPT Inhibitor FK866 in Myeloid Leukemic Cells. Int. J. Cancer 2013, 132, 766–774. [Google Scholar] [CrossRef]
- Schuster, S.; Penke, M.; Gorski, T.; Gebhardt, R.; Weiss, T.S.; Kiess, W.; Garten, A. FK866-induced NAMPT inhibition activates AMPK and downregulates mTOR signaling in hepatocarcinoma cells. Biochem. Biophys. Res. Commun. 2015, 458, 334–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Xia, L.; Li, F.; Zhou, L.; Weng, Q.; Li, Z.; Wu, Y.; Mao, Y.; Zhang, C.; Wu, Y.; et al. mTOR complexes differentially orchestrates eosinophil development in allergy. Sci. Rep. 2018, 8, 6883. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Frank, A.R.; Jewell, J.L. mTOR signaling in stem and progenitor cells. Development 2018, 145, dev152595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grolla, A.A.; Torretta, S.; Gnemmi, I.; Amoruso, A.; Orsomando, G.; Gatti, M.; Caldarelli, A.; Lim, D.; Penengo, L.; Brunelleschi, S.; et al. Nicotinamide phosphoribosyltransferase (NAMPT/PBEF/visfatin) is a tumoural cytokine released from melanoma. Pigment. Cell Melanoma Res. 2015, 28, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Orkin, S.H.; Zon, L.I. Hematopoiesis: An evolving paradigm for stem cell biology. Cell 2008, 132, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Y.; Liu, R.; Ikenoue, T.; Guan, K.L.; Liu, Y.; Zheng, P. TSC-MTOR Maintains Quiescence and Function of Hematopoietic Stem Cells by Repressing Mitochondrial Biogenesis and Reactive Oxygen Species. J. Exp. Med. 2008, 205, 2397–2408. [Google Scholar] [CrossRef]
- Geest, C.R.; Zwartkruis, F.J.; Vellenga, E.; Coffer, P.J.; Buitenhuis, M. Mammalian Target of Rapamycin Activity Is Required for Expansion of CD34+ Hematopoietic Progenitor Cells. Haematologica 2009, 94, 901–910. [Google Scholar] [CrossRef]
- Nahimana, A.; Attinger, A.; Aubry, D.; Greaney, P.; Ireson, C.; Thougaard, A.V.; Tjørnelund, J.; Dawson, K.M.; Dupuis, M.; Duchosal, M.A. The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood 2009, 113, 3276–3286. [Google Scholar] [CrossRef]
- Travelli, C.; Colombo, G.; Mola, S.; Genazzani, A.A.; Porta, C. NAMPT: A pleiotropic modulator of monocytes and macrophages. Pharmacol. Res. 2018, 135, 25–36. [Google Scholar] [CrossRef]
- Camp, S.M.; Ceco, E.; Evenoski, C.L.; Danilov, S.M.; Zhou, T.; Chiang, E.T.; Moreno-Vinasco, L.; Mapes, B.; Zhao, J.; Gursoy, G.; et al. Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NFkappaB Signaling and Inflammatory Lung Injury. Sci. Rep. 2015, 5, 13135. [Google Scholar] [CrossRef]
- Nagai, Y.; Garrett, K.P.; Ohta, S.; Bahrun, U.; Kouro, T.; Akira, S.; Takatsu, K.; Kincade, P.W. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity 2006, 24, 801–812. [Google Scholar] [CrossRef]
- Holmes, C.; Stanford, W.L. Concise Review: Stem Cell Antigen-1: Expression, Function, and Enigma. Stem Cells 2007, 25, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Siggins, R.W.; Stanford, W.L.; Melvan, J.N.; Basson, M.D.; Zhang, P. Toll-Like Receptor 4/Stem Cell Antigen 1 Signaling Promotes Hematopoietic Precursor Cell Commitment to Granulocyte Development During the Granulopoietic Response to Escherichia Coli Bacteremia. Infect. Immun. 2013, 81, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.; Leport, M.; Szilagyi, C.; Allen, J.M.; Bertrand, C.; Lagente, V. DMSO-Treated HL60 Cells: A Model of Neutrophil-Like Cells Mainly Expressing PDE4B Subtype. Int. Immunopharmacol. 2002, 2, 1647–1656. [Google Scholar] [CrossRef]
- Kanayasu-Toyoda, T.; Yamaguchi, T.; Uchida, E.; Hayakawa, T. Commitment of Neutrophilic ifferentiation and Proliferation of HL-60 Cells Coincides With Expression of Transferrin Receptor. Effect of Granulocyte Colony Stimulating Factor on Differentiation and Proliferation. J. Biol. Chem. 1999, 274, 25471–25480. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siakaeva, E.; Pylaeva, E.; Spyra, I.; Bordbari, S.; Höing, B.; Kürten, C.; Lang, S.; Jablonska, J. Neutrophil Maturation and Survival Is Controlled by IFN-Dependent Regulation of NAMPT Signaling. Int. J. Mol. Sci. 2019, 20, 5584. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225584
Siakaeva E, Pylaeva E, Spyra I, Bordbari S, Höing B, Kürten C, Lang S, Jablonska J. Neutrophil Maturation and Survival Is Controlled by IFN-Dependent Regulation of NAMPT Signaling. International Journal of Molecular Sciences. 2019; 20(22):5584. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225584
Chicago/Turabian StyleSiakaeva, Elena, Ekaterina Pylaeva, Ilona Spyra, Sharareh Bordbari, Benedikt Höing, Cornelius Kürten, Stephan Lang, and Jadwiga Jablonska. 2019. "Neutrophil Maturation and Survival Is Controlled by IFN-Dependent Regulation of NAMPT Signaling" International Journal of Molecular Sciences 20, no. 22: 5584. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225584




