Start Codon Recognition in Eukaryotic and Archaeal Translation Initiation: A Common Structural Core
Abstract
:1. Introduction
2. Features of Eukaryotic and Archaeal Translation Initiation Factors
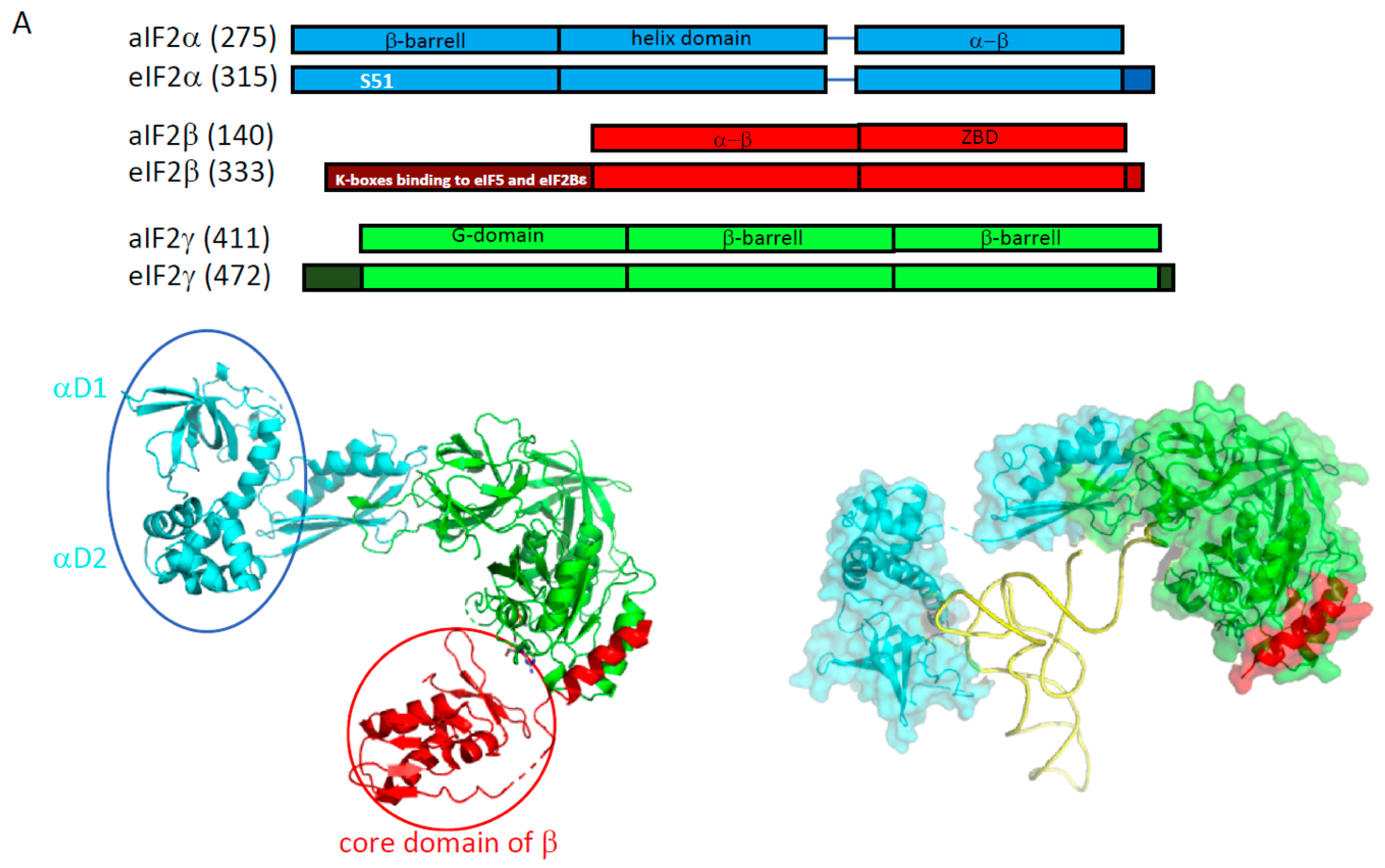
2.1. e/aIF2
2.1.1. e/aIF2-tRNA Complex
2.1.2. Nucleotide Cycle on e/aIF2
2.1.3. Regulation of eIF2 Assembly by Cdc123
2.2. e/aIF1A
2.3. e/aIF1
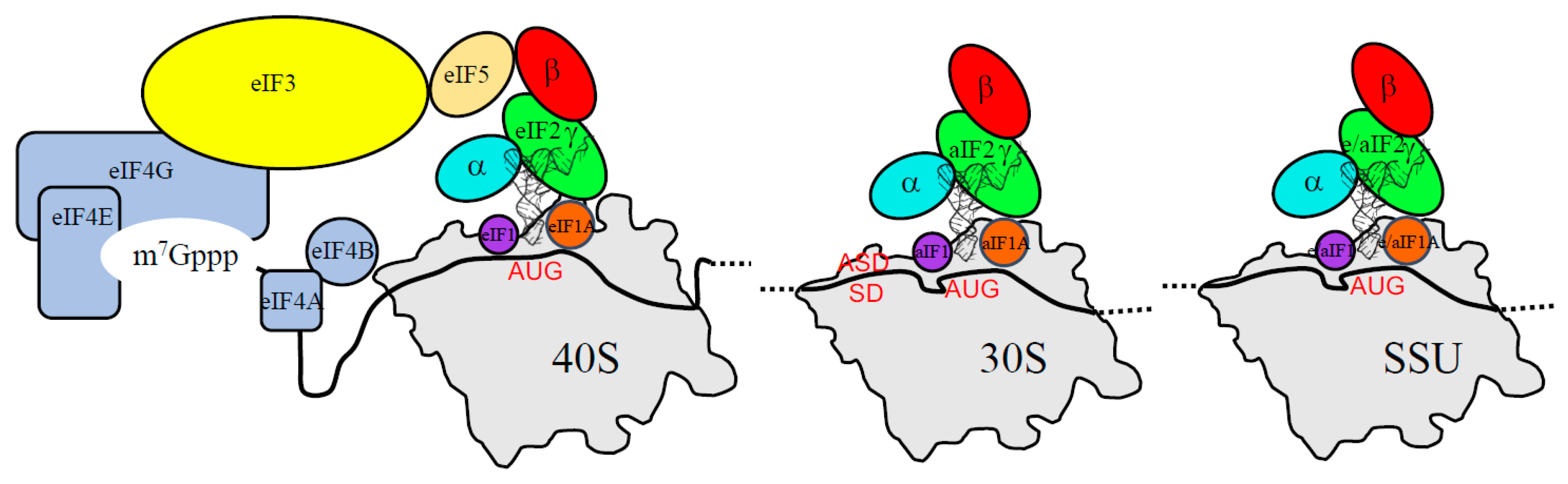
3. The Three Initiation Factors in the PIC
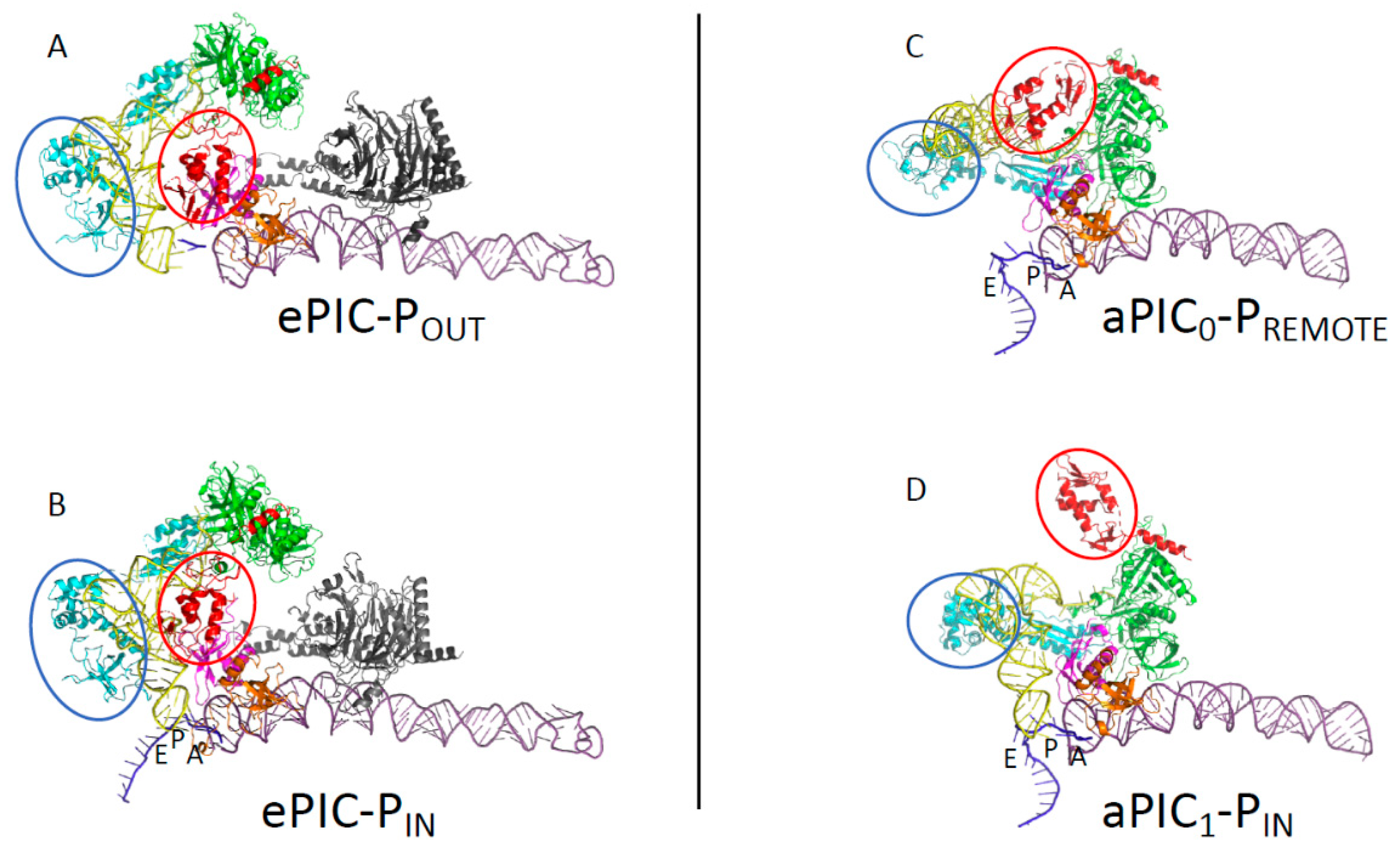
3.1. The Scanning Model in Eukaryotes
3.2. Control of AUG Selection by aIF2 in Archaea
4. Evolution of Translation Initiation Mechanisms and Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PIC | pre-initiation complex |
| e/aIF2 | Eukaryotic/archaeal initiation factor 2 |
| TC | Ternary complex eIF2-GTP-Met-tRNAiMet |
| LUCA | Last Universal Common Ancestor |
References
- Woese, C.R.; Gutell, R.; Gupta, R.; Noller, H.F. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol. Rev. 1983, 47, 621–669. [Google Scholar] [PubMed]
- Woese, C.R.; Fox, G.E. Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proc. Natl. Acad. Sci. USA 1977, 74, 5088–5090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woese, C.R.; Kandler, O.; Wheelis, M.L. Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl. Acad. Sci. USA 1990, 87, 4576–4579. [Google Scholar] [CrossRef] [PubMed]
- Hartman, H.; Favaretto, P.; Smith, T.F. The archaeal origins of the eukaryotic translational system. Archaea 2006, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyrpides, N.C.; Woese, C.R. Universally conserved translation initiation factors. Proc. Natl. Acad. Sci. USA 1998, 95, 224–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyrpides, N.C.; Woese, C.R. Archaeal translation initiation revisited: The initiation factor 2 and eukaryotic initiation factor 2B alpha-beta-delta subunit families. Proc. Natl. Acad. Sci. USA 1998, 95, 3726–3730. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.; Oakes, M.; Clark, M.W.; Lake, J.A.; Matheson, A.T.; Zillig, W. A new ribosome structure. Science 1984, 225, 510–512. [Google Scholar] [CrossRef]
- Lake, J.A. Origin of the eukaryotic nucleus determined by rate-invariant analysis of rRNA sequences. Nature 1988, 331, 184–186. [Google Scholar] [CrossRef]
- Lake, J.A.; Henderson, E.; Oakes, M.; Clark, M.W. Eocytes: A new ribosome structure indicates a kingdom with a close relationship to eukaryotes. Proc. Natl. Acad. Sci. USA 1984, 81, 3786–3790. [Google Scholar] [CrossRef]
- Rivera, M.C.; Lake, J.A. Evidence that eukaryotes and eocyte prokaryotes are immediate relatives. Science 1992, 257, 74–76. [Google Scholar] [CrossRef]
- Cox, C.J.; Foster, P.G.; Hirt, R.P.; Harris, S.R.; Embley, T.M. The archaebacterial origin of eukaryotes. Proc. Natl. Acad. Sci. USA 2008, 105, 20356–20361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Roberts, D. On the use of nucleic acid sequences to infer early branchings in the tree of life. Mol. Biol. Evol. 1995, 12, 451–458. [Google Scholar] [PubMed]
- Tourasse, N.J.; Gouy, M. Accounting for evolutionary rate variation among sequence sites consistently changes universal phylogenies deduced from rRNA and protein-coding genes. Mol. Phylogenet. Evol. 1999, 13, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Spang, A.; Eme, L.; Saw, J.H.; Caceres, E.F.; Zaremba-Niedzwiedzka, K.; Lombard, J.; Guy, L.; Ettema, T.J.G. Asgard archaea are the closest prokaryotic relatives of eukaryotes. PLoS Genet. 2018, 14, e1007080. [Google Scholar] [CrossRef] [PubMed]
- Zaremba-Niedzwiedzka, K.; Caceres, E.F.; Saw, J.H.; Backstrom, D.; Juzokaite, L.; Vancaester, E.; Seitz, K.W.; Anantharaman, K.; Starnawski, P.; Kjeldsen, K.U.; et al. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 2017, 541, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, V.; Gaia, M.; Nasir, A.; Forterre, P. Asgard archaea do not close the debate about the universal tree of life topology. PLoS Genet. 2018, 14, e1007215. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Foster, P.G.; Cox, C.J.; Embley, T.M. An archaeal origin of eukaryotes supports only two primary domains of life. Nature 2013, 504, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Koonin, E.V.; Yutin, N. The dispersed archaeal eukaryome and the complex archaeal ancestor of eukaryotes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016188. [Google Scholar] [CrossRef]
- Castelle, C.J.; Banfield, J.F. Major New Microbial Groups Expand Diversity and Alter our Understanding of the Tree of Life. Cell 2018, 172, 1181–1197. [Google Scholar] [CrossRef]
- Forterre, P. The universal tree of life: An update. Front. Microbiol. 2015, 6, 717. [Google Scholar] [CrossRef]
- Harish, A. What is an archaeon and are the Archaea really unique? PeerJ 2018, 6, e5770. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, H.; Philippe, H. Archaea sister group of Bacteria? Indications from tree reconstruction artifacts in ancient phylogenies. Mol. Biol. Evol. 1999, 16, 817–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forterre, P.; Philippe, H. Where is the root of the universal tree of life? Bioessays 1999, 21, 871–879. [Google Scholar] [CrossRef]
- Mayr, E. Two empires or three? Proc. Natl. Acad. Sci. USA 1998, 95, 9720–9723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurland, C.G.; Harish, A. Structural biology and genome evolution: An introduction. Biochimie 2015, 119, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Harish, A.; Kurland, C.G. Akaryotes and Eukaryotes are independent descendants of a universal common ancestor. Biochimie 2017, 138, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Lecompte, O.; Ripp, R.; Thierry, J.C.; Moras, D.; Poch, O. Comparative analysis of ribosomal proteins in complete genomes: An example of reductive evolution at the domain scale. Nucleic Acids Res. 2002, 30, 5382–5390. [Google Scholar] [CrossRef] [PubMed]
- Ban, N.; Beckmann, R.; Cate, J.H.; Dinman, J.D.; Dragon, F.; Ellis, S.R.; Lafontaine, D.L.; Lindahl, L.; Liljas, A.; Lipton, J.M.; et al. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014, 24, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Z.; Whitman, W.B. Evolution of the archaeal and mammalian information processing systems: Towards an archaeal model for human disease. Cell. Mol. Life Sci. 2017, 74, 183–212. [Google Scholar] [CrossRef]
- Borck, G.; Shin, B.S.; Stiller, B.; Mimouni-Bloch, A.; Thiele, H.; Kim, J.R.; Thakur, M.; Skinner, C.; Aschenbach, L.; Smirin-Yosef, P.; et al. eIF2gamma mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation. Mol. Cell 2012, 48, 641–646. [Google Scholar] [CrossRef]
- Moortgat, S.; Desir, J.; Benoit, V.; Boulanger, S.; Pendeville, H.; Nassogne, M.C.; Lederer, D.; Maystadt, I. Two novel EIF2S3 mutations associated with syndromic intellectual disability with severe microcephaly, growth retardation, and epilepsy. Am. J. Med. Genet. Part A 2016, 170, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Skopkova, M.; Hennig, F.; Shin, B.S.; Turner, C.E.; Stanikova, D.; Brennerova, K.; Stanik, J.; Fischer, U.; Henden, L.; Muller, U.; et al. EIF2S3 Mutations Associated with Severe X-Linked Intellectual Disability Syndrome MEHMO. Hum. Mutat. 2017, 38, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, S.E.; Stolboushkina, E.A.; Terenin, I.M.; Andreev, D.E.; Garber, M.B.; Shatsky, I.N. Archaeal translation initiation factor aIF2 can substitute for eukaryotic eIF2 in ribosomal scanning during mammalian 48S complex formation. J. Mol. Biol. 2011, 413, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Perrochia, L.; Crozat, E.; Hecker, A.; Zhang, W.; Bareille, J.; Collinet, B.; van Tilbeurgh, H.; Forterre, P.; Basta, T. In vitro biosynthesis of a universal t6A tRNA modification in Archaea and Eukarya. Nucleic Acids Res. 2013, 41, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. Structural Insights into the Mechanism of Scanning and Start Codon Recognition in Eukaryotic Translation Initiation. Trends Biochem. Sci. 2017, 42, 589–611. [Google Scholar] [CrossRef] [PubMed]
- Shirokikh, N.E.; Preiss, T. Translation initiation by cap-dependent ribosome recruitment: Recent insights and open questions. Wiley Interdiscip. Rev. RNA 2018, 9, e1473. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Barends, S.; Jaeger, S.; Schaeffer, L.; Prongidi-Fix, L.; Eriani, G. Cap-assisted internal initiation of translation of histone H4. Mol. Cell 2011, 41, 197–209. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Shatsky, I.N.; Terenin, I.M.; Smirnova, V.V.; Andreev, D.E. Cap-Independent Translation: What’s in a Name? Trends Biochem. Sci. 2018, 43, 882–895. [Google Scholar] [CrossRef]
- Coots, R.A.; Liu, X.M.; Mao, Y.; Dong, L.; Zhou, J.; Wan, J.; Zhang, X.; Qian, S.B. m(6)A Facilitates eIF4F-Independent mRNA Translation. Mol. Cell 2017, 68, 504.e7–514.e7. [Google Scholar] [CrossRef]
- Akulich, K.A.; Andreev, D.E.; Terenin, I.M.; Smirnova, V.V.; Anisimova, A.S.; Makeeva, D.S.; Arkhipova, V.I.; Stolboushkina, E.A.; Garber, M.B.; Prokofjeva, M.M.; et al. Four translation initiation pathways employed by the leaderless mRNA in eukaryotes. Sci. Rep. 2016, 6, 37905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, P.P. Ancient ciphers: Translation in Archaea. Cell 1997, 89, 1007–1010. [Google Scholar] [CrossRef]
- Gogoi, P.; Srivastava, A.; Jayaprakash, P.; Jeyakanthan, J.; Kanaujia, S.P. In silico analysis suggests that PH0702 and PH0208 encode for methylthioribose-1-phosphate isomerase and ribose-1,5-bisphosphate isomerase, respectively, rather than aIF2Bbeta and aIF2Bdelta. Gene 2016, 575, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Dev, K.; Santangelo, T.J.; Rothenburg, S.; Neculai, D.; Dey, M.; Sicheri, F.; Dever, T.E.; Reeve, J.N.; Hinnebusch, A.G. Archaeal aIF2B interacts with eukaryotic translation initiation factors eIF2alpha and eIF2Balpha: Implications for aIF2B function and eIF2B regulation. J. Mol. Biol. 2009, 392, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Naveau, M.; Mechulam, Y. Eukaryotic and archaeal translation initiation factor 2: A heterotrimeric tRNA carrier. FEBS Lett. 2010, 584, 405–412. [Google Scholar] [CrossRef]
- Kapp, L.D.; Kolitz, S.E.; Lorsch, J.R. Yeast initiator tRNA identity elements cooperate to influence multiple steps of translation initiation. RNA 2006, 12, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Kapp, L.D.; Lorsch, J.R. GTP-dependent Recognition of the Methionine Moiety on Initiator tRNA by Translation Factor eIF2. J. Mol. Biol. 2004, 335, 923–936. [Google Scholar] [CrossRef]
- Yatime, L.; Mechulam, Y.; Blanquet, S.; Schmitt, E. Structural switch of the gamma subunit in an archaeal aIF2 alpha gamma heterodimer. Structure 2006, 14, 119–128. [Google Scholar] [CrossRef]
- Yatime, L.; Schmitt, E.; Blanquet, S.; Mechulam, Y. Functional molecular mapping of archaeal translation initiation factor 2. J. Biol. Chem. 2004, 279, 15984–15993. [Google Scholar] [CrossRef]
- Marck, C.; Grosjean, H. tRNomics: Analysis of tRNA genes from 50 genomes of Eukarya, Archaea, and Bacteria reveals anticodon-sparing strategies and domain-specific features. RNA 2002, 8, 1189–1232. [Google Scholar] [CrossRef] [Green Version]
- Monestier, A.; Aleksandrov, A.; Coureux, P.D.; Panvert, M.; Mechulam, Y.; Schmitt, E. The structure of an E. coli tRNAfMet A1-U72 variant shows an unusual conformation of the A1-U72 base pair. RNA 2017, 23, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Cigan, A.M.; Feng, L.; Donahue, T.F. tRNAi(met) functions in directing the scanning ribosome to the start site of translation. Science 1988, 242, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Cigan, A.M.; Pabich, E.K.; Feng, L.; Donahue, T.F. Yeast translation initiation suppressor sui2 encodes the alpha subunit of eukaryotic initiation factor 2 and shares sequence identity with the human alpha subunit. Proc. Natl. Acad. Sci. USA 1989, 86, 2784–2788. [Google Scholar] [CrossRef]
- Dorris, D.R.; Erickson, F.L.; Hannig, E.M. Mutations in GCD11, the structural gene for eIF-2 gamma in yeast, alter translational regulation of GCN4 and the selection of the start site for protein synthesis. EMBO J. 1995, 14, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Harashima, S.; Hinnebusch, A.G. Multiple GCD genes required for repression of GCN4, a transcriptional activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 1986, 6, 3990–3998. [Google Scholar] [CrossRef]
- Donahue, T.F.; Cigan, A.M. Genetic selection for mutations that reduce or abolish ribosomal recognition of the HIS4 translational initiator region. Mol. Cell. Biol. 1988, 8, 2955–2963. [Google Scholar] [CrossRef]
- Pathak, V.K.; Nielsen, P.J.; Trachsel, H.; Hershey, J.W. Structure of the beta subunit of translational initiation factor eIF-2. Cell 1988, 54, 633–639. [Google Scholar] [CrossRef]
- Huang, H.K.; Yoon, H.; Hannig, E.M.; Donahue, T.F. GTP hydrolysis controls stringent selection of the AUG start codon during translation initiation in Saccharomyces cerevisiae. Genes Dev. 1997, 11, 2396–2413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannig, E.M.; Cigan, A.M.; Freeman, B.A.; Kinzy, T.G. GCD11, a negative regulator of GCN4 expression, encodes the gamma subunit of eIF-2 in Saccharomyces cerevisiae. Mol. Cell. Biol. 1993, 13, 506–520. [Google Scholar] [CrossRef]
- Erickson, F.L.; Hannig, E.M. Ligand interactions with eukaryotic translation initiation factor 2: Role of the gamma-subunit. EMBO J. 1996, 15, 6311–6320. [Google Scholar] [CrossRef]
- Roll-Mecak, A.; Alone, P.; Cao, C.; Dever, T.E.; Burley, S.K. X-ray structure of translation initiation factor eIF2gamma: Implications for tRNA and eIF2alpha binding. J. Biol. Chem. 2004, 279, 10634–10642. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Blanquet, S.; Mechulam, Y. The large subunit of initiation factor aIF2 is a close structural homologue of elongation factors. EMBO J. 2002, 21, 1821–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokabe, M.; Yao, M.; Sakai, N.; Toya, S.; Tanaka, I. Structure of archaeal translational initiation factor 2 betagamma-GDP reveals significant conformational change of the beta-subunit and switch 1 region. Proc. Natl. Acad. Sci. USA 2006, 103, 13016–13021. [Google Scholar] [CrossRef] [PubMed]
- Stolboushkina, E.; Nikonov, S.; Nikulin, A.; Blasi, U.; Manstein, D.J.; Fedorov, R.; Garber, M.; Nikonov, O. Crystal structure of the intact archaeal translation initiation factor 2 demonstrates very high conformational flexibility in the alpha- and beta-subunits. J. Mol. Biol. 2008, 382, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Nikonov, O.; Stolboushkina, E.; Arkhipova, V.; Kravchenko, O.; Nikonov, S.; Garber, M. Conformational transitions in the gamma subunit of the archaeal translation initiation factor 2. Acta Crystallogr. D Biol. Crystallogr. 2014, 70 Pt 3, 658–667. [Google Scholar] [CrossRef]
- Yatime, L.; Mechulam, Y.; Blanquet, S.; Schmitt, E. Structure of an archaeal heterotrimeric initiation factor 2 reveals a nucleotide state between the GTP and the GDP states. Proc. Natl. Acad. Sci. USA 2007, 104, 18445–18450. [Google Scholar] [CrossRef]
- Dubiez, E.; Aleksandrov, A.; Lazennec-Schurdevin, C.; Mechulam, Y.; Schmitt, E. Identification of a second GTP-bound magnesium ion in archaeal initiation factor 2. Nucleic Acids Res. 2015, 43, 2946–2957. [Google Scholar] [CrossRef]
- Yatime, L.; Schmitt, E.; Blanquet, S.; Mechulam, Y. Structure-function relationships of the intact aIF2a subunit from the archaeon Pyrococcus abyssi. Biochemistry 2005, 44, 8749–8756. [Google Scholar] [CrossRef]
- Schmitt, E.; Panvert, M.; Lazennec-Schurdevin, C.; Coureux, P.D.; Perez, J.; Thompson, A.; Mechulam, Y. Structure of the ternary initiation complex aIF2-GDPNP-methionylated initiator tRNA. Nat. Struct. Mol. Biol. 2012, 19, 450–454. [Google Scholar] [CrossRef]
- Nissen, P.; Kjeldgaard, M.; Thirup, S.; Polekhina, G.; Reshetnikova, L.; Clark, B.F.C.; Nyborg, J. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science 1995, 270, 1464–1472. [Google Scholar] [CrossRef]
- Pedulla, N.; Palermo, R.; Hasenohrl, D.; Blasi, U.; Cammarano, P.; Londei, P. The archaeal eIF2 homologue: Functional properties of an ancient translation initiation factor. Nucleic Acids Res. 2005, 33, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Monestier, A.; Lazennec-Schurdevin, C.; Coureux, P.D.; Mechulam, Y.; Schmitt, E. Role of aIF1 in Pyrococcus abyssi translation initiation. Nucleic Acids Res. 2018, 46, 11061–11074. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Hinnebusch, A.G. eIF1 Loop 2 interactions with Met-tRNAi control the accuracy of start codon selection by the scanning preinitiation complex. Proc. Natl. Acad. Sci. USA 2018, 115, E4159–E4168. [Google Scholar] [PubMed]
- Nika, J.; Rippel, S.; Hannig, E.M. Biochemical analysis of the eIF2bg complex reveals a structural function for eIF2a in catalyzed nucleotide exchange. J. Biol. Chem. 2001, 276, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Naveau, M.; Lazennec-Schurdevin, C.; Panvert, M.; Dubiez, E.; Mechulam, Y.; Schmitt, E. Roles of yeast eIF2alpha and eIF2beta subunits in the binding of the initiator methionyl-tRNA. Nucleic Acids Res. 2013, 41, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Naveau, M.; Lazennec-Schurdevin, C.; Panvert, M.; Mechulam, Y.; Schmitt, E. tRNA binding properties of eukaryotic translation initiation factor 2 from Encephalitozoon cuniculi. Biochemistry 2010, 49, 8680–8688. [Google Scholar] [CrossRef] [PubMed]
- Stolboushkina, E.; Nikonov, S.; Zelinskaya, N.; Arkhipova, V.; Nikulin, A.; Garber, M.; Nikonov, O. Crystal structure of the archaeal translation initiation factor 2 in complex with a GTP analogue and Met-tRNAf(Met.). J. Mol. Biol. 2013, 425, 989–998. [Google Scholar] [CrossRef]
- Konieczny, A.; Safer, B. Purification of the eukaryotic initiation factor 2-eukaryotic initiation factor 2B complex and characterization of its guanine nucleotide exchange activity during protein synthesis initiation. J. Biol. Chem. 1983, 258, 3402–3408. [Google Scholar]
- Vazquez de Aldana, C.R.; Hinnebusch, A.G. Mutations in the GCD7 subunit of yeast guanine nucleotide exchange factor eIF-2B overcome the inhibitory effects of phosphorylated eIF-2 on translation initiation. Mol. Cell. Biol. 1994, 14, 3208–3222. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Takahashi, M.; Nishimoto, M.; Hiyama, T.B.; Higo, T.; Umehara, T.; Sakamoto, K.; Ito, T.; Yokoyama, S. Crystal structure of eukaryotic translation initiation factor 2B. Nature 2016, 531, 122–125. [Google Scholar] [CrossRef]
- Zyryanova, A.F.; Weis, F.; Faille, A.; Alard, A.A.; Crespillo-Casado, A.; Sekine, Y.; Harding, H.P.; Allen, F.; Parts, L.; Fromont, C.; et al. Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B. Science 2018, 359, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Krishnamoorthy, T.; Phan, L.; Pavitt, G.D.; Hinnebusch, A.G. Conserved bipartite motifs in yeast eIF5 and eIF2Bepsilon, GTPase-activating and GDP-GTP exchange factors in translation initiation, mediate binding to their common substrate eIF2. EMBO J. 1999, 18, 1673–1688. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Maiti, T.; Das, K.; Maitra, U. Specific interaction of eukaryotic translation initiation factor 5 (eIF5) with the beta-subunit of eIF2. J. Biol. Chem. 1997, 272, 31712–31718. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Zhang, Y.; Zeng, H.; Novoa, I.; Lu, P.D.; Calfon, M.; Sadri, N.; Yun, C.; Popko, B.; Paules, R.; et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 2003, 11, 619–633. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [Green Version]
- Pavitt, G.D. eIF2B, a mediator of general and gene-specific translational control. Biochem. Soc. Trans. 2005, 33 Pt 6, 1487–1492. [Google Scholar] [CrossRef] [Green Version]
- Wortham, N.C.; Proud, C.G. eIF2B: Recent structural and functional insights into a key regulator of translation. Biochem. Soc. Trans. 2015, 43, 1234–1240. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Translational regulation of GCN4 and the general amino acid control of yeast. Annu. Rev. Microbiol. 2005, 59, 407–450. [Google Scholar] [CrossRef]
- Lu, P.D.; Harding, H.P.; Ron, D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004, 167, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Terenin, I.M.; Dmitriev, S.E.; Andreev, D.E.; Shatsky, I.N. Eukaryotic translation initiation machinery can operate in a bacterial-like mode without eIF2. Nat. Struct. Mol. Biol. 2008, 15, 836–841. [Google Scholar] [CrossRef]
- Thakor, N.; Holcik, M. IRES-mediated translation of cellular messenger RNA operates in eIF2alpha- independent manner during stress. Nucleic Acids Res. 2012, 40, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Pestova, T.V.; de Breyne, S.; Pisarev, A.V.; Abaeva, I.S.; Hellen, C.U. eIF2-dependent and eIF2-independent modes of initiation on the CSFV IRES: A common role of domain II. EMBO J. 2008, 27, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Evidence for translational regulation of the activator of general amino acid control in yeast. Proc. Natl. Acad. Sci. USA 1984, 81, 6442–6446. [Google Scholar] [CrossRef] [PubMed]
- Thireos, G.; Penn, M.D.; Greer, H. 5’ untranslated sequences are required for the translational control of a yeast regulatory gene. Proc. Natl. Acad. Sci. USA 1984, 81, 5096–5100. [Google Scholar] [CrossRef] [PubMed]
- Schleich, S.; Strassburger, K.; Janiesch, P.C.; Koledachkina, T.; Miller, K.K.; Haneke, K.; Cheng, Y.S.; Kuechler, K.; Stoecklin, G.; Duncan, K.E.; et al. DENR-MCT-1 promotes translation re-initiation downstream of uORFs to control tissue growth. Nature 2014, 512, 208–212. [Google Scholar] [CrossRef]
- Dmitriev, S.E.; Terenin, I.M.; Andreev, D.E.; Ivanov, P.A.; Dunaevsky, J.E.; Merrick, W.C.; Shatsky, I.N. GTP-independent tRNA delivery to the ribosomal P-site by a novel eukaryotic translation factor. J. Biol. Chem. 2010, 285, 26779–26787. [Google Scholar] [CrossRef]
- Kim, E.; Kim, J.H.; Seo, K.; Hong, K.Y.; An, S.W.A.; Kwon, J.; Lee, S.V.; Jang, S.K. eIF2A, an initiator tRNA carrier refractory to eIF2alpha kinases, functions synergistically with eIF5B. Cell. Mol. Life Sci. 2018, 75, 4287–4300. [Google Scholar] [CrossRef]
- Ho, J.J.D.; Balukoff, N.C.; Cervantes, G.; Malcolm, P.D.; Krieger, J.R.; Lee, S. Oxygen-Sensitive Remodeling of Central Carbon Metabolism by Archaic eIF5B. Cell Rep. 2018, 22, 17–26. [Google Scholar] [CrossRef]
- Ross, J.; Bressler, K.; Thakor, N. Eukaryotic Initiation Factor 5B (eIF5B) Cooperates with eIF1A and eIF5 to Facilitate uORF2-Mediated Repression of ATF4 Translation. Int. J. Mol. Sci. 2018, 19, 4032. [Google Scholar] [CrossRef]
- Mechulam, Y.; Blanquet, S.; Schmitt, E. Translation Initiation. EcoSal Plus 2011, 4. [Google Scholar] [CrossRef]
- Tahara, M.; Ohsawa, A.; Saito, S.; Kimura, M. In vitro phosphorylation of initiation factor 2 alpha (aIF2 alpha) from hyperthermophilic archaeon Pyrococcus horikoshii OT3. J. Biochem. 2004, 135, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Wurgler-Murphy, S.M.; King, D.M.; Kennelly, P.J. The Phosphorylation Site Database: A guide to the serine-, threonine-, and/or tyrosine-phosphorylated proteins in prokaryotic organisms. Proteomics 2004, 4, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Benelli, D.; Londei, P. Translation initiation in Archaea: Conserved and domain-specific features. Biochem. Soc. Trans. 2011, 39, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Bieganowski, P.; Shilinski, K.; Tsichlis, P.N.; Brenner, C. Cdc123 and checkpoint forkhead associated with RING proteins control the cell cycle by controlling eIF2gamma abundance. J. Biol. Chem. 2004, 279, 44656–44666. [Google Scholar] [CrossRef]
- Perzlmaier, A.F.; Richter, F.; Seufert, W. Translation initiation requires cell division cycle 123 (Cdc123) to facilitate biogenesis of the eukaryotic initiation factor 2 (eIF2). J. Biol. Chem. 2013, 288, 21537–21546. [Google Scholar] [CrossRef] [PubMed]
- Panvert, M.; Dubiez, E.; Arnold, L.; Perez, J.; Mechulam, Y.; Seufert, W.; Schmitt, E. Cdc123, a Cell Cycle Regulator Needed for eIF2 Assembly, Is an ATP-Grasp Protein with Unique Features. Structure 2015, 23, 1596–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreev, D.E.; Dmitriev, S.E.; Loughran, G.; Terenin, I.M.; Baranov, P.V.; Shatsky, I.N. Translation control of mRNAs encoding mammalian translation initiation factors. Gene 2018, 651, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, H.; Erni, B.; Schreier, M.H.; Staehelin, T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J. Mol. Biol. 1977, 116, 755–767. [Google Scholar] [CrossRef]
- Thomas, A.A.; Benne, R.; Voorma, H.O. Initiation of eukaryotic protein synthesis. FEBS Lett. 1981, 128, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Dever, T.E.; Wei, C.L.; Benkowski, L.A.; Browning, K.; Merrick, W.C.; Hershey, J.W. Determination of the amino acid sequence of rabbit, human, and wheat germ protein synthesis factor eIF-4C by cloning and chemical sequencing. J. Biol. Chem. 1994, 269, 3212–3218. [Google Scholar] [PubMed]
- Wei, C.L.; MacMillan, S.E.; Hershey, J.W. Protein synthesis initiation factor eIF-1A is a moderately abundant RNA-binding protein. J. Biol. Chem. 1995, 270, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Battiste, J.L.; Pestova, T.V.; Hellen, C.U.; Wagner, G. The eIF1A solution structure reveals a large RNA-binding surface important for scanning function. Mol. Cell 2000, 5, 109–119. [Google Scholar] [CrossRef]
- Yu, Y.; Marintchev, A.; Kolupaeva, V.G.; Unbehaun, A.; Veryasova, T.; Lai, S.C.; Hong, P.; Wagner, G.; Hellen, C.U.; Pestova, T.V. Position of eukaryotic translation initiation factor eIF1A on the 40S ribosomal subunit mapped by directed hydroxyl radical probing. Nucleic Acids Res. 2009, 37, 5167–5182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coureux, P.D.; Lazennec-Schurdevin, C.; Monestier, A.; Larquet, E.; Cladiere, L.; Klaholz, B.P.; Schmitt, E.; Mechulam, Y. Cryo-EM study of start codon selection during archaeal translation initiation. Nat. Commun. 2016, 7, 13366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Llacer, J.L.; Fernandez, I.S.; Munoz, A.; Martin-Marcos, P.; Savva, C.G.; Lorsch, J.R.; Hinnebusch, A.G.; Ramakrishnan, V. Structural changes enable start codon recognition by the eukaryotic translation initiation complex. Cell 2014, 159, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Weisser, M.; Voigts-Hoffmann, F.; Rabl, J.; Leibundgut, M.; Ban, N. The crystal structure of the eukaryotic 40S ribosomal subunit in complex with eIF1 and eIF1A. Nat. Struct. Mol. Biol. 2013, 20, 1015–1017. [Google Scholar] [CrossRef]
- Lomakin, I.B.; Steitz, T.A. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature 2013, 500, 307–311. [Google Scholar] [CrossRef]
- Castilho-Valavicius, B.; Yoon, H.; Donahue, T.F. Genetic characterization of the Saccharomyces cerevisiae translational initiation suppressors sui1, sui2 and SUI3 and their effects on HIS4 expression. Genetics 1990, 124, 483–495. [Google Scholar]
- Fletcher, C.M.; Pestova, T.V.; Hellen, C.U.; Wagner, G. Structure and interactions of the translation initiation factor eIF1. EMBO J. 1999, 18, 2631–2637. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, P.; Kanaujia, S.P. Archaeal and eukaryal translation initiation factor 1 differ in their RNA interacting loops. FEBS Lett. 2018, 592, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Lomakin, I.B.; Kolupaeva, V.G.; Marintchev, A.; Wagner, G.; Pestova, T.V. Position of eukaryotic initiation factor eIF1 on the 40S ribosomal subunit determined by directed hydroxyl radical probing. Genes Dev. 2003, 17, 2786–2797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabl, J.; Leibundgut, M.; Ataide, S.F.; Haag, A.; Ban, N. Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1. Science 2011, 331, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Pestova, T.V.; Borukhov, S.I.; Hellen, C.U. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature 1998, 394, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Passmore, L.A.; Schmeing, T.M.; Maag, D.; Applefield, D.J.; Acker, M.G.; Algire, M.A.; Lorsch, J.R.; Ramakrishnan, V. The Eukaryotic Translation Initiation Factors eIF1 and eIF1A Induce an Open Conformation of the 40S Ribosome. Mol. Cell 2007, 26, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Maag, D.; Lorsch, J.R. Communication between eukaryotic translation initiation factors 1 and 1A on the yeast small ribosomal subunit. J. Mol. Biol. 2003, 330, 917–924. [Google Scholar] [CrossRef]
- Chaudhuri, J.; Si, K.; Maitra, U. Function of eukaryotic translation initiation factor 1A (eIF1A) (formerly called eIF-4C) in initiation of protein synthesis. J. Biol. Chem. 1997, 272, 7883–7891. [Google Scholar] [CrossRef] [PubMed]
- Algire, M.A.; Maag, D.; Savio, P.; Acker, M.G.; Tarun, S.Z., Jr.; Sachs, A.B.; Asano, K.; Nielsen, K.H.; Olsen, D.S.; Phan, L.; et al. Development and characterization of a reconstituted yeast translation initiation system. RNA 2002, 8, 382–397. [Google Scholar] [CrossRef] [Green Version]
- Fekete, C.A.; Applefield, D.J.; Blakely, S.A.; Shirokikh, N.; Pestova, T.; Lorsch, J.R.; Hinnebusch, A.G. The eIF1A C-terminal domain promotes initiation complex assembly, scanning and AUG selection in vivo. EMBO J. 2005, 24, 3588–3601. [Google Scholar] [CrossRef] [Green Version]
- Fekete, C.A.; Mitchell, S.F.; Cherkasova, V.A.; Applefield, D.; Algire, M.A.; Maag, D.; Saini, A.K.; Lorsch, J.R.; Hinnebusch, A.G. N- and C-terminal residues of eIF1A have opposing effects on the fidelity of start codon selection. EMBO J. 2007, 26, 1602–1614. [Google Scholar] [CrossRef] [Green Version]
- Saini, A.K.; Nanda, J.S.; Lorsch, J.R.; Hinnebusch, A.G. Regulatory elements in eIF1A control the fidelity of start codon selection by modulating tRNA(i)(Met) binding to the ribosome. Genes Dev. 2010, 24, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.F.; Lorsch, J.R. Should I stay or should I go? Eukaryotic translation initiation factors 1 and 1A control start codon recognition. J. Biol. Chem. 2008, 283, 27345–27349. [Google Scholar] [CrossRef] [PubMed]
- Algire, M.A.; Maag, D.; Lorsch, J.R. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 2005, 20, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Llacer, J.L.; Hussain, T.; Marler, L.; Aitken, C.E.; Thakur, A.; Lorsch, J.R.; Hinnebusch, A.G.; Ramakrishnan, V. Conformational Differences between Open and Closed States of the Eukaryotic Translation Initiation Complex. Mol. Cell 2015, 59, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Martin-Marcos, P.; Nanda, J.; Luna, R.E.; Wagner, G.; Lorsch, J.R.; Hinnebusch, A.G. beta-hairpin loop of eIF1 mediates 40S ribosome binding to regulate initiator tRNAMet recruitment and accuracy of AUG selection in vivo. J. Biol. Chem. 2013, 27546–27562. [Google Scholar] [CrossRef] [PubMed]
- des Georges, A.; Dhote, V.; Kuhn, L.; Hellen, C.U.; Pestova, T.V.; Frank, J.; Hashem, Y. Structure of mammalian eIF3 in the context of the 43S preinitiation complex. Nature 2015, 525, 491–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashem, Y.; des Georges, A.; Dhote, V.; Langlois, R.; Liao, H.Y.; Grassucci, R.A.; Hellen, C.U.; Pestova, T.V.; Frank, J. Structure of the mammalian ribosomal 43S preinitiation complex bound to the scanning factor DHX29. Cell 2013, 153, 1108–1119. [Google Scholar] [CrossRef]
- Pisarev, A.V.; Kolupaeva, V.G.; Pisareva, V.P.; Merrick, W.C.; Hellen, C.U.; Pestova, T.V. Specific functional interactions of nucleotides at key -3 and +4 positions flanking the initiation codon with components of the mammalian 48S translation initiation complex. Genes Dev. 2006, 20, 624–636. [Google Scholar] [CrossRef] [Green Version]
- Hinnebusch, A.G. eIF3: A versatile scaffold for translation initiation complexes. Trends Biochem. Sci. 2006, 31, 553–562. [Google Scholar] [CrossRef]
- Hashem, Y.; Frank, J. The Jigsaw Puzzle of mRNA Translation Initiation in Eukaryotes: A Decade of Structures Unraveling the Mechanics of the Process. Annu. Rev. Biophys. 2018. [Google Scholar] [CrossRef]
- Valasek, L.S.; Zeman, J.; Wagner, S.; Beznoskova, P.; Pavlikova, Z.; Mohammad, M.P.; Hronova, V.; Herrmannova, A.; Hashem, Y.; Gunisova, S. Embraced by eIF3: Structural and functional insights into the roles of eIF3 across the translation cycle. Nucleic Acids Res. 2017, 45, 10948–10968. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, A.; Brito Querido, J.; Myasnikov, A.G.; Mancera-Martinez, E.; Renaud, A.; Kuhn, L.; Hashem, Y. eIF3 Peripheral Subunits Rearrangement after mRNA Binding and Start-Codon Recognition. Mol. Cell 2016, 63, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Nag, N.; Pestova, T.V.; Marintchev, A. Human eIF5 and eIF1A Compete for Binding to eIF5B. Biochemistry 2018, 57, 5910–5920. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.S.; Kim, J.R.; Walker, S.E.; Dong, J.; Lorsch, J.R.; Dever, T.E. Initiation factor eIF2gamma promotes eIF2-GTP-Met-tRNAi(Met) ternary complex binding to the 40S ribosome. Nat. Struct. Mol. Biol. 2011, 18, 1227–1234. [Google Scholar] [CrossRef]
- Hasenöhrl, D.; Benelli, D.; Barbazza, A.; Londei, P.; Bläsi, U. Sulfolobus solfataricus translation initiation factor 1 stimulates translation initiation complex formation. RNA 2006, 12, 674–682. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.K.; Lee, J.H.; Zoll, W.L.; Merrick, W.C.; Dever, T.E. Promotion of met-tRNAiMet binding to ribosomes by yIF2, a bacterial IF2 homolog in yeast. Science 1998, 280, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Pestova, T.V.; Lomakin, I.B.; Lee, J.H.; Choi, S.K.; Dever, T.E.; Hellen, C.U. The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature 2000, 403, 332–335. [Google Scholar] [CrossRef]
- Roll-Mecak, A.; Cao, C.; Dever, T.E.; Burley, S.K. X-Ray structures of the universal translation initiation factor IF2/eIF5B: Conformational changes on GDP and GTP binding. Cell 2000, 103, 781–792. [Google Scholar] [CrossRef]
- Gualerzi, C.O.; Pon, C.L. Initiation of mRNA translation in bacteria: Structural and dynamic aspects. Cell. Mol. Life Sci. 2015, 72, 4341–4367. [Google Scholar] [CrossRef]
- Rodnina, M.V. Translation in Prokaryotes. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef]
- Beck, H.J.; Moll, I. Leaderless mRNAs in the Spotlight: Ancient but Not Outdated! Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Gogoi, P.; Deka, B.; Goswami, S.; Kanaujia, S.P. In silico analysis of 5’-UTRs highlights the prevalence of Shine-Dalgarno and leaderless-dependent mechanisms of translation initiation in bacteria and archaea, respectively. J. Theor. Biol. 2016, 402, 54–61. [Google Scholar] [CrossRef]
- Torarinsson, E.; Klenk, H.P.; Garrett, R.A. Divergent transcriptional and translational signals in Archaea. Environ. Microbiol. 2005, 7, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup, N.; Sensen, C.W.; Garrett, R.A.; Clausen, I.G. Two different and highly organized mechanisms of translation initiation in the archaeon Sulfolobus solfataricus. Extremophiles 2000, 4, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Wurtzel, O.; Sapra, R.; Chen, F.; Zhu, Y.; Simmons, B.A.; Sorek, R. A single-base resolution map of an archaeal transcriptome. Genome Res. 2010, 20, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Condo, I.; Ciammaruconi, A.; Benelli, D.; Ruggero, D.; Londei, P. Cis-acting signals controlling translational initiation in the thermophilic archaeon Sulfolobus solfataricus. Mol. Microbiol. 1999, 34, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babski, J.; Haas, K.A.; Nather-Schindler, D.; Pfeiffer, F.; Forstner, K.U.; Hammelmann, M.; Hilker, R.; Becker, A.; Sharma, C.M.; Marchfelder, A.; et al. Genome-wide identification of transcriptional start sites in the haloarchaeon Haloferax volcanii based on differential RNA-Seq (dRNA-Seq). BMC Genomics 2016, 17, 629. [Google Scholar] [CrossRef]
- Toffano-Nioche, C.; Ott, A.; Crozat, E.; Nguyen, A.N.; Zytnicki, M.; Leclerc, F.; Forterre, P.; Bouloc, P.; Gautheret, D. RNA at 92 degrees C: The non-coding transcriptome of the hyperthermophilic archaeon Pyrococcus abyssi. RNA Biol. 2013, 10, 1211–1220. [Google Scholar] [CrossRef]
- Benelli, D.; Maone, E.; Londei, P. Two different mechanisms for ribosome/mRNA interaction in archaeal translation initiation. Mol. Microbiol. 2003, 50, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Moll, I.; Hirokawa, G.; Kiel, M.C.; Kaji, A.; Blasi, U. Translation initiation with 70S ribosomes: An alternative pathway for leaderless mRNAs. Nucleic Acids Res. 2004, 32, 3354–3363. [Google Scholar] [CrossRef]
- Grill, S.; Gualerzi, C.O.; Londei, P.; Blasi, U. Selective stimulation of translation of leaderless mRNA by initiation factor 2: Evolutionary implications for translation. EMBO J. 2000, 19, 4101–4110. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G.; Ivanov, I.P.; Sonenberg, N. Translational control by 5’-untranslated regions of eukaryotic mRNAs. Science 2016, 352, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Brenneis, M.; Hering, O.; Lange, C.; Soppa, J. Experimental characterization of Cis-acting elements important for translation and transcription in halophilic archaea. PLoS Genet. 2007, 3, e229. [Google Scholar] [CrossRef] [PubMed]
- Thiaville, P.C.; El Yacoubi, B.; Kohrer, C.; Thiaville, J.J.; Deutsch, C.; Iwata-Reuyl, D.; Bacusmo, J.M.; Armengaud, J.; Bessho, Y.; Wetzel, C.; et al. Essentiality of threonylcarbamoyladenosine (t(6)A), a universal tRNA modification, in bacteria. Mol. Microbiol. 2015, 98, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Akil, C.; Robinson, R.C. Genomes of Asgard archaea encode profilins that regulate actin. Nature 2018, 562, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Eme, L.; Ettema, T.J.G. The eukaryotic ancestor shapes up. Nature 2018, 562, 352–353. [Google Scholar] [CrossRef] [PubMed]



| Eukaryotes | Archaea | Bacteria |
|---|---|---|
| mRNA features | ||
| Canonical capped dependent, Kozak motif Non-canonical capped dependent Non-canonical capped independent IRES-mediated Leaderless | SD-dependent Leaderless | SD-dependent Leaderless |
| main initiator tRNA features | ||
| methionine A1-U72 G29-C41, G30-C40, G31-C39 | methionine A1-U72 G29-C41, G30-C40, G31-C39 | formyl-methionine mispaired 1-72 bases G29-C41, G30-C40, G31-C39 |
| Translation initiation factors | ||
| eIF2 (α,β,γ) | aIF2 (α,β,γ) | - |
| eIF1 | aIF1 | ~IF3 * |
| eIF1A | aIF1A | IF1 |
| eIF5B § | aIF5B § | IF2 |
| eIF5 | - | - |
| eIF2B (α,β,γ,δ,ε) | aIF2B (α,β,δ) # | - |
| eIF3 (6 to 13 subunits) | - | - |
| eIF4F (4A, 4G, 4E) | aIF4A ! | - |
| eIF4B | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitt, E.; Coureux, P.-D.; Monestier, A.; Dubiez, E.; Mechulam, Y. Start Codon Recognition in Eukaryotic and Archaeal Translation Initiation: A Common Structural Core. Int. J. Mol. Sci. 2019, 20, 939. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040939
Schmitt E, Coureux P-D, Monestier A, Dubiez E, Mechulam Y. Start Codon Recognition in Eukaryotic and Archaeal Translation Initiation: A Common Structural Core. International Journal of Molecular Sciences. 2019; 20(4):939. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040939
Chicago/Turabian StyleSchmitt, Emmanuelle, Pierre-Damien Coureux, Auriane Monestier, Etienne Dubiez, and Yves Mechulam. 2019. "Start Codon Recognition in Eukaryotic and Archaeal Translation Initiation: A Common Structural Core" International Journal of Molecular Sciences 20, no. 4: 939. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20040939






