1. Introduction
Elevated urinary albumin excretion (UAE) is well known to be associated with cardiovascular disease (CVD) risk [
1,
2,
3,
4,
5,
6,
7,
8]. Furthermore, it is likely that coexisting atherogenic lipoprotein abnormalities in individuals with elevated UAE modify CVD risk in this setting [
9,
10], a notion that is consistent with reports of elevated levels of plasma atherogenic apolipoprotein B (apoB) particles in individuals with proteinuria [
11,
12,
13,
14,
15]. In this case, elevated UAE and atherogenic apoB particles may act independently or together in modulating CVD risk. If acting in concert, this might be indicative of shared pathogenic mechanisms leading to CVD risk. Thus, in an earlier report from the Prevention of Renal and Vascular Endstage Disease (PREVEND) study [
10], statistical interactions of elevated UAE with multiple apoB-containing lipoprotein fractions were demonstrated, leading to the conclusion that elevated UAE and atherogenic apoB lipoproteins characteristic of albuminuric subjects likely do share common pathogenic mechanisms leading to CVD.
In contrast, for high-density lipoprotein (HDL) in individuals with nephrotic syndrome and chronic kidney disease (CKD), HDL cholesterol (HDL-C) is within or below normal limits [
16]. Additionally, in these individuals, profound changes in the structure and protective features of HDL particles may occur, including impairments in reverse cholesterol transport, as well as anti-inflammatory, antioxidant, and antithrombotic functionalities [
16]. Accordingly, in the current study, we hypothesized that various attributes of HDL particles might demonstrate modulation of the CVD risk associated with elevated UAE, which is potentially indicative of a commonality of pathogenic pathways. To address this question, we investigated 6,286 participants of the PREVEND study using nuclear magnetic resonance (NMR) lipoprotein analyses with a particular focus on HDL-associated parameters along with multivariable Cox proportional hazards risk modeling as a function of UAE and HDL-associated parameters by NMR to determine risk associations and potential interactions between UAE and HDL-associated parameters that might underlie CVD risk.
3. Discussion
The current work set-up as a dual-phase study utilizing multivariable risk modeling investigated incident CVD in a cohort of individuals of the PREVEND study as a function of UAE and measures of apoA-I content of HDL particles and concentrations of size-based HDL subfractions. In addition to confirming previous findings of risk associated with elevated UAE [
1,
2,
3,
4,
5,
6,
7,
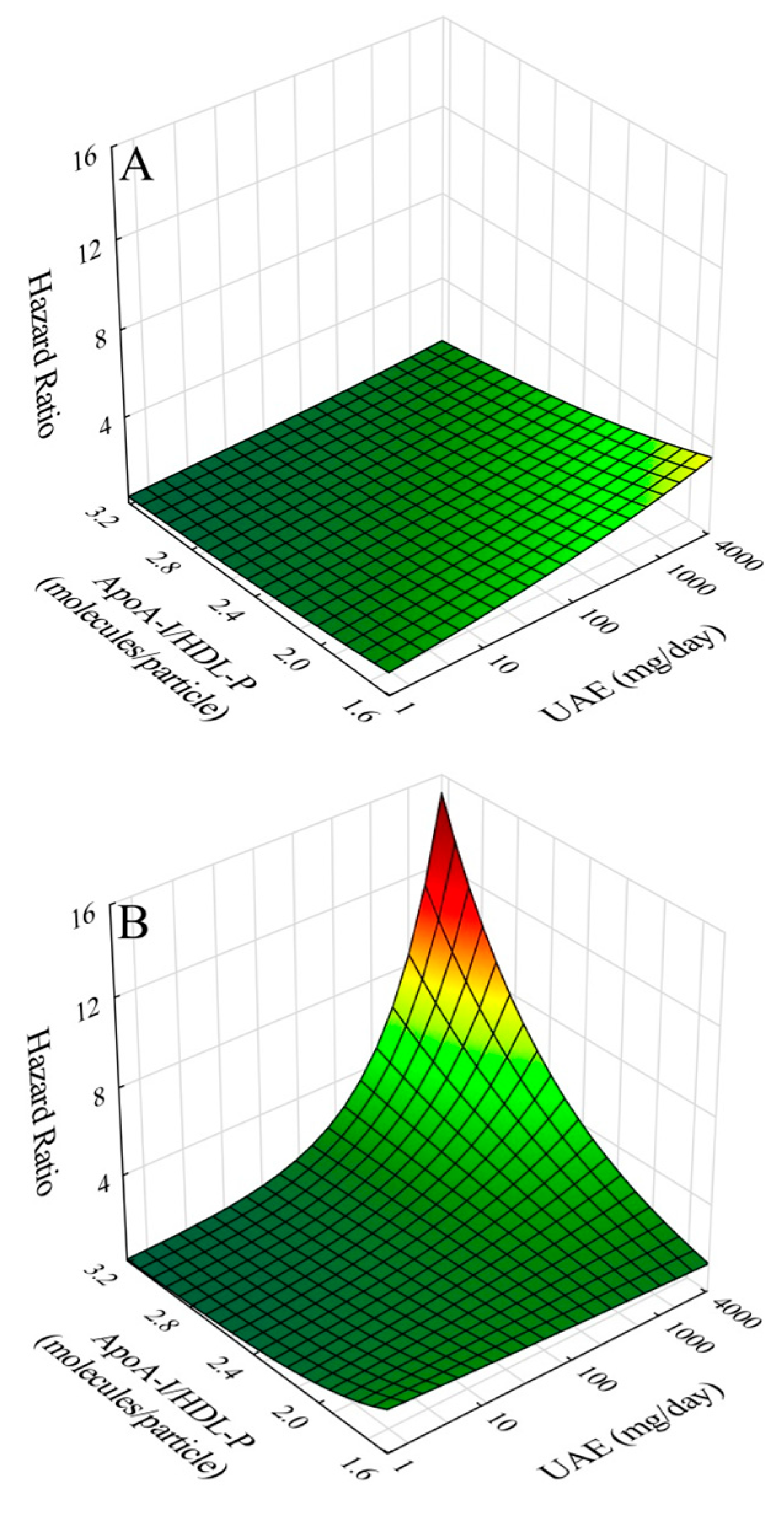
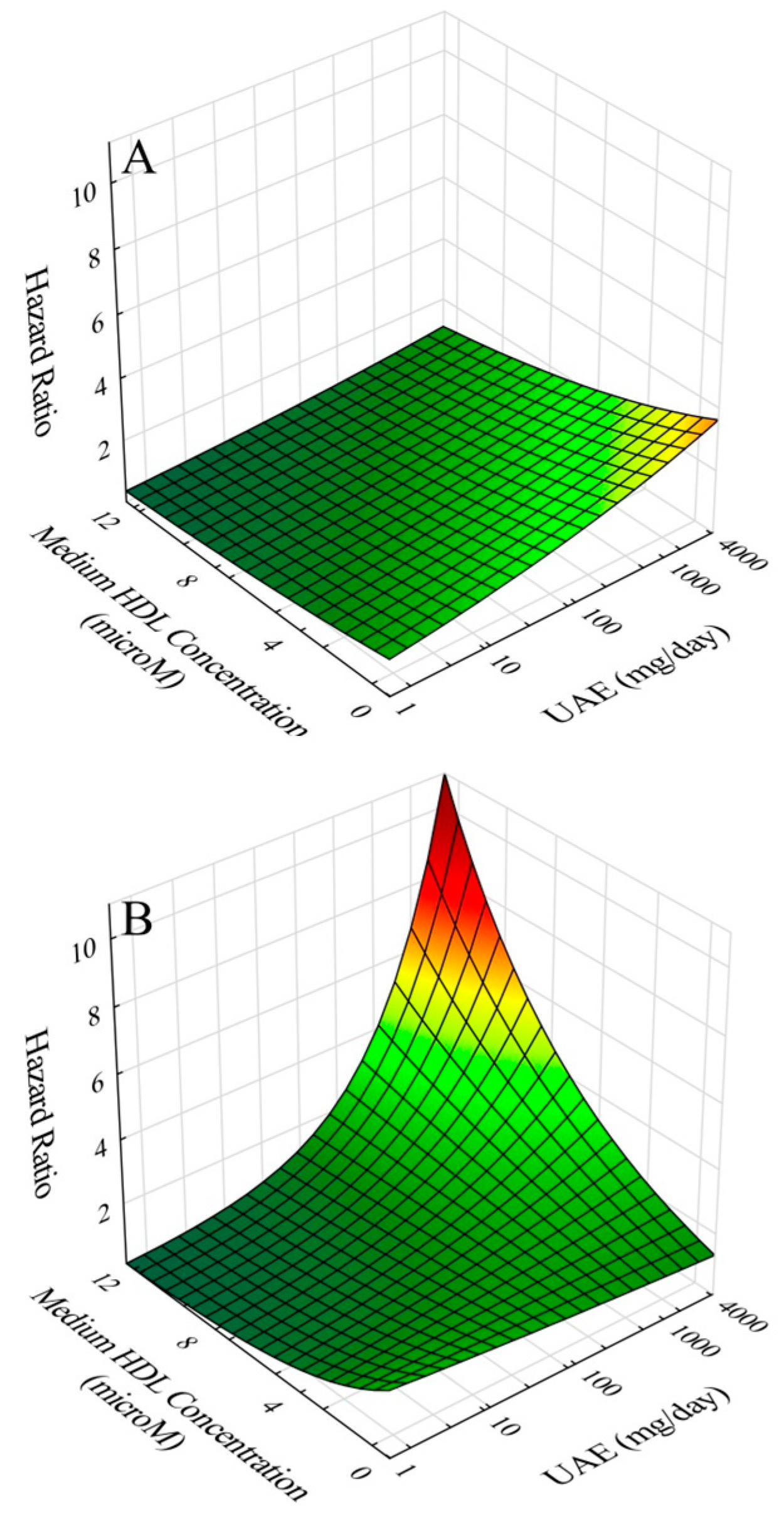
8], baseline screening results using standard lipid and lipoprotein analyses revealed significant risk associations for HDL-C, apoA-I, and apoA-I/HDL-C. These findings were subsequently confirmed and extended using NMR lipoprotein analyses on samples collected four years later in risk models adjusted for gender, age, apoB, diabetes, past CVD history, CRP and eGFR. The results revealed an inverse association with risk for HDL-C, apoA-I, HDL-P, apoA-I/HDL-P, large HDL-P, and medium HDL-P, and a direct association for apoA-I/HDL-C. Moreover, in terms of interactions, further modeling revealed significant interactions between UAE and apoA-I/HDL-P and between UAE and medium HDL-P. In both cases, inclusion of interaction terms in risk models resulted in amplified risk at concurrently high levels of UAE and apoA-I/HDL-P and at concurrently high levels of UAE and concentration of medium-size HDL particles. We interpret these results to be consistent with our hypothesis that compositional features of HDL particles modulate the CVD risk associated with elevated UAE; furthermore, such findings may be indicative of pathogenic mechanisms shared by high UAE and compositional features of HDL particles in the development of CVD risk.
The results of our study demonstrated an independent protective effects against CVD risk for apoA-I/HDL-P. However, upon interaction with elevated UAE, the risk increased. This reversal is likely related to alterations in HDL structure and function in the setting of proteinuria resulting in pro-atherogenic transformation of HDL, especially as related to reverse cholesterol transport (RCT) [
16]. Multiple factors associated with proteinuria have been shown to contribute to pro-atherogenic alterations in HDL that affect RCT by impairing maturation of HDL particles, particularly as related to cholesteryl ester enrichment. These include: 1. deficiency of lecithin-cholesteryl acyltransferase (LCAT) [
17] as LCAT together with apoA-I mediates esterification of free cholesterol, although elevated LCAT activity has also been reported in proteinuria [
18]; 2. hypoalbuminemia as albumin delivers significant amounts of free cholesterol from peripheral tissues to HDL particles, as it is a sign of inflammation [
19]; 3. increased levels of cholesterol ester transfer protein (CETP) resulting in depletion of HDL particle cholesterol esters [
20]; 4. decreased levels of the SRB1 hepatic HDL receptor resulting in decreased hepatic uptake of cholesteryl ester [
21]; and 5. increased levels of the hepatic HDL endocytic receptor resulting in decreased apoA-I and HDL levels via increase in apoA-I mediated uptake of lipid-poor HDL [
22]. In addition to proteinuria-associated RCT impairment, other atheroprotective HDL functions including anti-oxidant, anti-inflammatory, and anti-thrombotic activities would also likely be compromised with elevated UAE as in chronic renal disease [
23,
24,
25]. Generation of dysfunctional HDL ensuing from these processes especially as affecting apoA-I would likely underlie the observed reversal of CVD risk association by apoA-I content of HDL from atheroprotective to atherogenic upon interaction with elevated UAE.
The results of our study also demonstrated independent protective effects against CVD risk by medium-size HDL particle concentration. However, again upon interaction with elevated UAE, the risk increased. Similar to apoA-I/HDL-P, medium HDL in the setting of elevated UAE could also develop into a source of pro-atherogenic HDL. Why this was not the case for concentrations of large and small HDL is not clear. Regarding large HDL, proportional hazards regression results with and without interaction were similar to those of medium HDL, except that the interaction term did not achieve statistical significance (p = 0.65) for large HDL. We speculate that this may relate to the generally greater estimated load of apoA-I carried by medium HDL particles (13.14 µM) in comparison to large HDL particles (8.12 µM) giving rise to a larger total pro-atherogenic load from medium HDL particles. The case for small-HDL particle concentration in UAE-associated risk was different in that interaction with elevated UAE did not achieve statistical significance.
In general consideration of HDL particle concentrations, the results of our study demonstrated that with increasing albuminuria, there were decreased concentrations of total, large, and medium size HDL particles, smaller HDL mean particle size, and increased concentration of small size HDL particles. These findings were generally consistent with the known lower levels of HDL
2 and higher levels of HDL
3 particles in nephrotic syndrome patients resulting from dysfunctional alterations of HDL secondary to impaired HDL particle maturation [
16,
26] and the lower total HDL particle concentration and lower mean HDL particle size in women with mild elevations in UAE [
9].
Limitations in our study should be acknowledged. Although analysis results in the baseline screening round indicated potential for the apoA-II content of HDL to play a role in UAE-associated CVD risk, apoA-II levels in the second screening round were not available for confirmation. Additionally, data relating to other HDL constituents that likely play major roles in HDL metabolic, anti-inflammatory, and anti-thrombotic functionality were not available for assessment of interaction with UAE in CVD risk. In addition, the nature of the study did not allow us to delineate causal relationships in the complicated interplay between elevated UAE and aspects of HDL functionality that contribute to the process of atherogenesis leading to CVD. Moreover, actual experimental data relating directly to changes in HDL particle structure, and especially function, were not available. However, our approach of suing statistical interactions to reveal effects of HDL compositional characteristics in the context of increasing UAE on risk can be anticipated to be helpful in select subpopulations of subjects, in which documentation of HDL functionality and metabolomics studies may be particularly insightful. The strengths of our study included a design in which preliminary findings determined at study initiation from a large prospective population-based study (N = 6,286) could be extended from results of a second round of data collection on the same subjects approximately four years after study initiation. Furthermore, the second round of data collection afforded NMR-based lipid and lipoprotein analyses, including determinations of mean HDL particle size and concentrations of HDL-C, apoA-I, and HDL particle subfractions (large, medium, and small), with the use of a state-of-the-art deconvolution algorithm for the NMR data.
To summarize, the current study was undertaken to determine whether HDL particles share common pathogenic pathways with elevated UAE in development of CVD risk. The study involved proportional hazards multivariable modeling on subjects of PREVEND, a large prospective population-based study, assessing risk as a function UAE and of HDL-associated parameters that were determined by NMR lipid and lipoprotein analyses. The results demonstrated not only significant risk associations for elevated UAE and for multiple HDL-associated parameters, but moreover, and importantly, significant interactions of elevated UAE with apoA-I/HDL-P, a measure of HDL apoA-I content, and with concentration of medium size HDL particles. We believe that our study findings are consistent with HDL particles sharing pathogenic pathways with elevated UAE in CVD risk. Future studies should be oriented toward pathway characterizations.