Cell Adhesion Molecule 1 Contributes to Cell Survival in Crowded Epithelial Monolayers
Abstract
:1. Introduction
2. Results
2.1. CADM1 Expression Increases as Epithelial Cells Crowd
2.2. CADM1 Knockdown Induces Apoptosis in Crowded Epithelial Cells
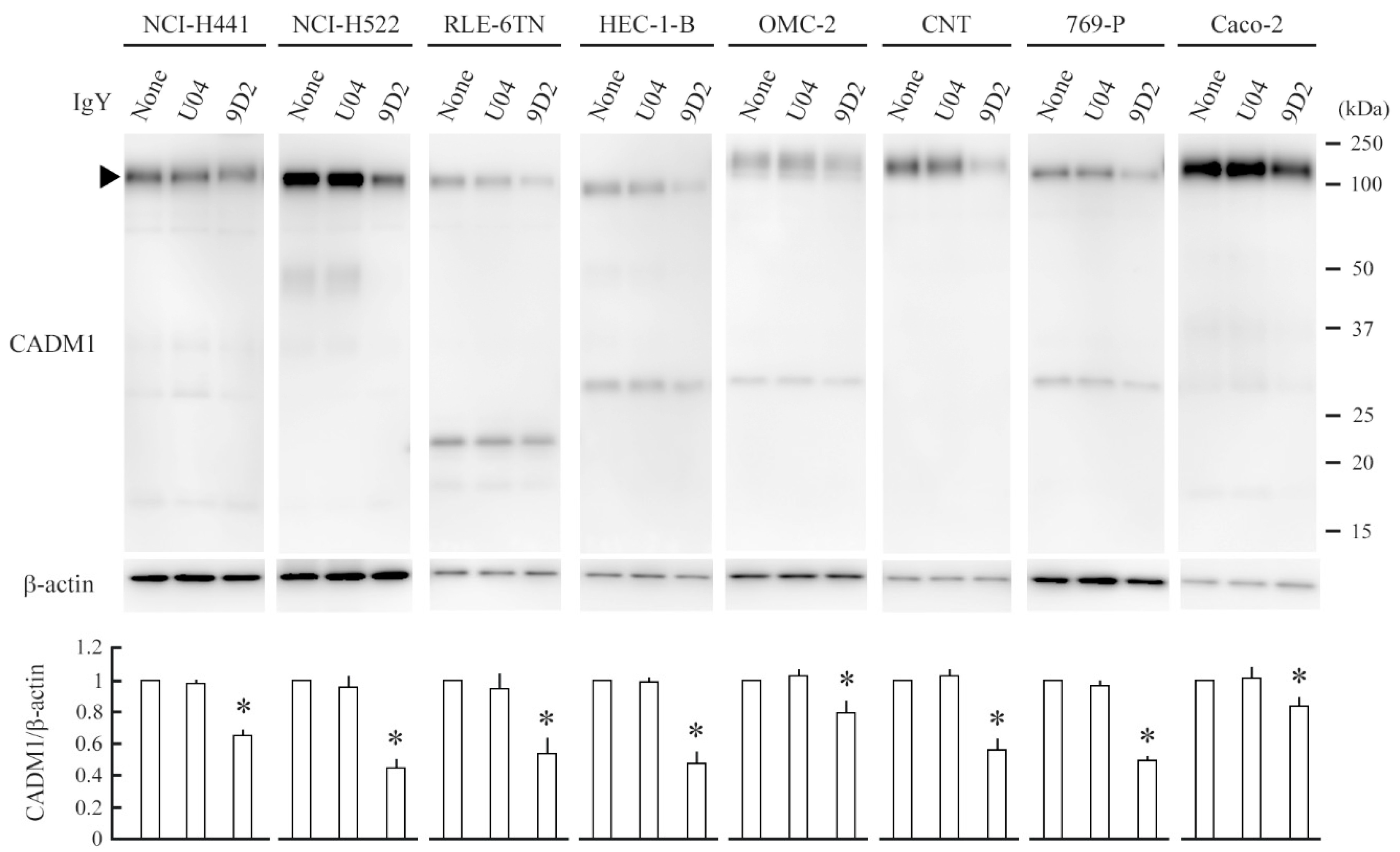
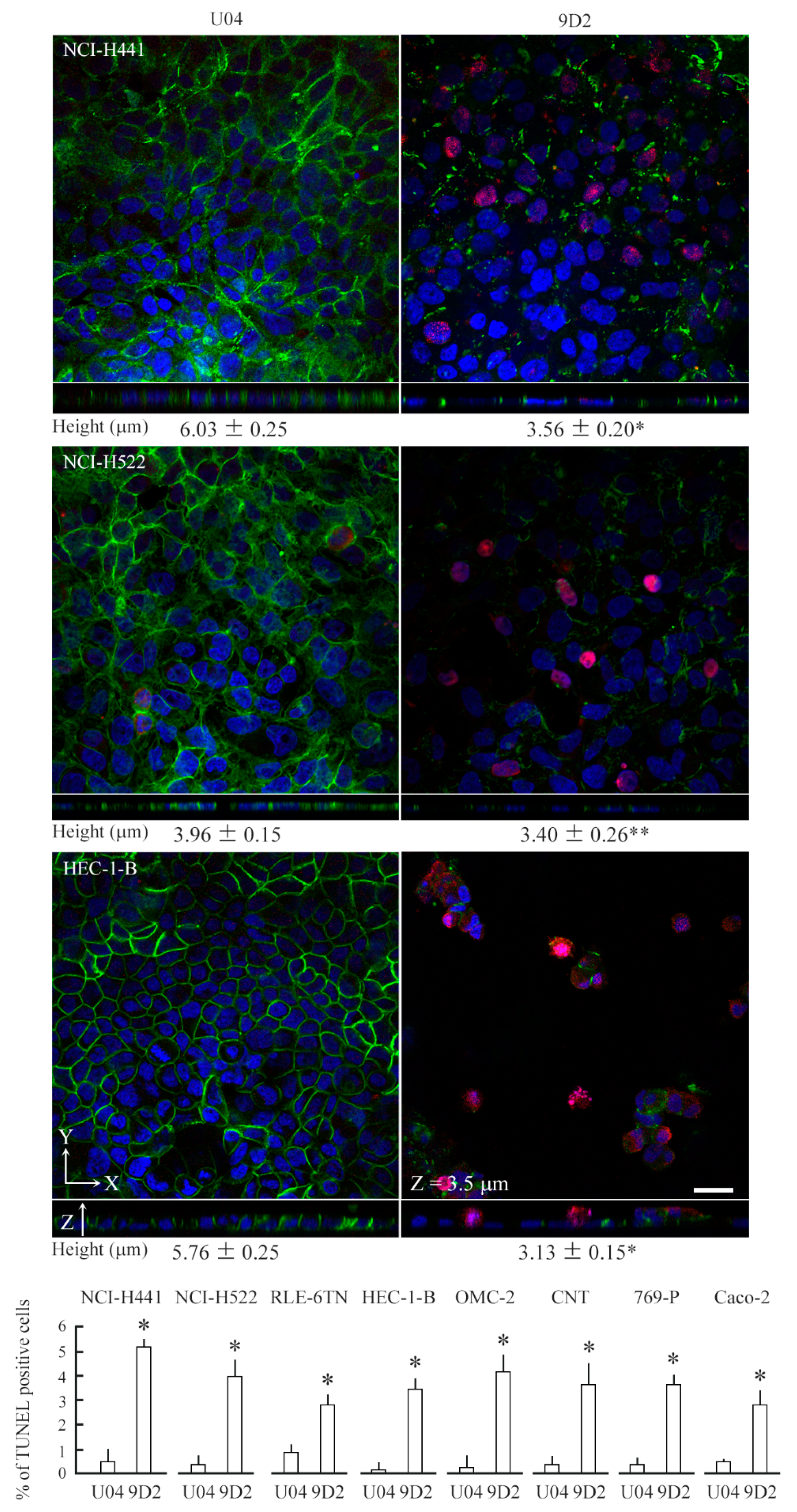
2.3. 9D2 Decreases CADM1 Levels and Induces Apoptosis in Crowded Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Antibodies
4.3. Cell Culture
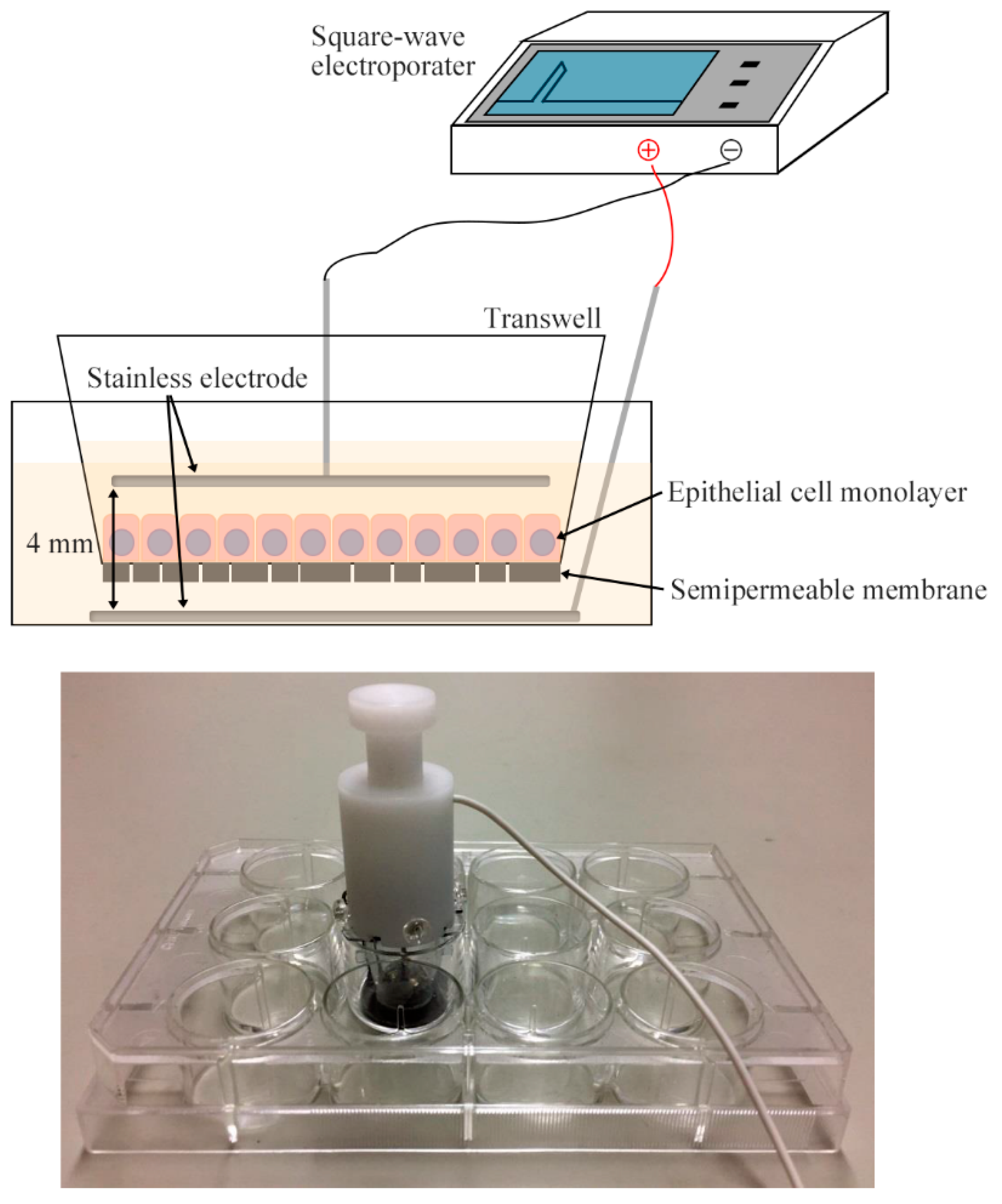
4.4. Electroporation
4.5. Western Blot Analysis
4.6. Immunofluorescence and Confocal Microscopy
4.7. 9D2 Treatment and Apoptosis Detection
4.8. RT-PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | 2-dimentional |
| ANOVA | analysis of variance |
| DAPI | 4′,6-diamidino-2-phenylindole |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| TUNEL | terminal deoxynucleotidyl transferase-mediated dNTP-biotin nick end labeling of DNA fragments |
References
- Tang, V. Cell-cell adhesion interface: Rise of the lateral membrane. F1000 Res. 2017, 6, 276. [Google Scholar] [CrossRef] [Green Version]
- Rikitake, Y.; Mandai, K.; Takai, Y. The role of nectins in different types of cell-cell adhesion. J. Cell Sci. 2012, 125, 3713–3722. [Google Scholar] [CrossRef] [Green Version]
- Ebnet, K.; Kummer, D.; Steinbacher, T.; Singh, A.; Nakayama, M.; Matis, M. Regulation of cell polarity by cell adhesion receptors. Semin. Cell Dev. Biol. 2018, 81, 2–12. [Google Scholar] [CrossRef]
- Eisenhoffer, G.T.; Loftus, P.D.; Yoshigi, M.; Otsuna, H.; Chien, C.B.; Morcos, P.A.; Rosenblatt, J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Hasleton, P.; Flieder, B.D. Spencer’s Pathology of the Lung, 6th ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- D’Agati, D.V.; Jennette, C.J.; Silva, G.F. Non-Neoplastic Kidney Diseases; King, W.D., Ed.; ARP: Washington, DC, USA, 2005; pp. 1–18. [Google Scholar]
- Oliva, E.; Wilbur, D.C.; Sebire, N.J.; Soslow, R.A. Tumors of the Uterine Corpus and Trophoblastic Diseases. In Atlas of Tumor Pathology, 4th ed.; Caton, Q.M., Thomas, S.D., Silva, C.M., Kahn, A., Tomlinson, E., Eds.; ARP: Arlington, VA, USA, 2020; pp. 3–13. [Google Scholar]
- Noffsinger, A.; Fenoglio-Preiser, M.C.; Maru, D.; Gilinsky, N. Gastrointestional Diseases; King, W.D., Ed.; ARP: Washington, DC, USA, 2007; pp. 1–31. [Google Scholar]
- Gumbiner, B.M.; Kim, N.G. The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 2014, 127, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhoffer, G.T.; Rosenblatt, J. Bringing balance by force: Live cell extrusion controls epithelial cell numbers. Trends Cell Biol. 2013, 23, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Xu, G.K.; Zhang, L.Y.; Gao, H. Stress-driven cell extrusion can maintain homeostatic cell density in response to overcrowding. Soft Matter 2019, 15, 8441–8449. [Google Scholar] [CrossRef]
- Ito, A.; Mimae, T.; Yamamoto, Y.S.Z.; Hagiyama, M.; Nakanishi, J.; Ito, M.; Hosokawa, Y.; Okada, M.; Murakami, Y.; Kondo, T. Novel application for pseudopodia proteomics using excimer laser ablation and two-dimensional difference gel electrophoresis. Lab. Investig. 2012, 92, 1374–1385. [Google Scholar] [CrossRef]
- Hagiyama, M.; Yabuta, N.; Okuzaki, D.; Inoue, T.; Takashima, Y.; Kimura, R.; Ri, A.; Ito, A. Modest Static Pressure Suppresses Columnar Epithelial Cell Growth in Association with Cell Shape and Cytoskeletal Modifications. Front Physiol. 2017, 8, 997. [Google Scholar] [CrossRef]
- Kuramochi, M.; Fukuhara, H.; Nobukuni, T.; Kanbe, T.; Maruyama, T.; Ghosh, H.P.; Pletcher, M.; Isomura, M.; Onizuka, M.; Kitamura, T.; et al. TSLC1 is a tumor-suppressor gene in human non-small-cell lung cancer. Nat. Genet. 2001, 27, 427–430. [Google Scholar] [CrossRef] [Green Version]
- Masuda, M.; Yageta, M.; Fukuhara, H.; Kuramochi, M.; Maruyama, T.; Nomoto, A.; Murakami, Y. The tumor suppressor protein TSLC1 is involved in cell-cell adhesion. J. Biol. Chem. 2002, 277, 31014–31019. [Google Scholar] [CrossRef] [Green Version]
- Shingai, T.; Ikeda, W.; Kakunaga, S.; Morimoto, K.; Takekuni, K.; Itoh, S.; Satoh, K.; Takeuchi, M.; Imai, T.; Monden, M.; et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J. Biol. Chem. 2003, 278, 35421–35427. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Okada, M.; Uchino, K.; Wakayama, T.; Koma, Y.; Iseki, S.; Tsubota, N.; Okita, Y.; Kitamura, Y. Expression of the TSLC1 adhesion molecule in pulmonary epithelium and its down-regulation in pulmonary adenocarcinoma other than bronchioloalveolar carcinoma. Lab. Investig. 2003, 83, 1175–1183. [Google Scholar] [CrossRef]
- Kato, T.; Hagiyama, M.; Takashima, Y.; Yoneshige, A.; Ito, A. Cell adhesion molecule-1 shedding induces apoptosis of renal epithelial cells and exacerbates human nephropathies. Am. J. Physiol. Ren. Physiol. 2018, 314, F388–F398. [Google Scholar] [CrossRef]
- Kimura, R.; Yoneshige, A.; Hagiyama, M.; Otani, T.; Inoue, T.; Shiraishi, N.; Yanagihara, K.; Wakayama, T.; Ito, A. Expression of cell adhesion molecule 1 in gastric neck and base glandular cells: Possible involvement in peritoneal dissemination of signet ring cells. Life Sci. 2018, 213, 206–213. [Google Scholar] [CrossRef]
- Sakurai-Yageta, M.; Masuda, M.; Tsuboi, Y.; Ito, A.; Murakami, Y. Tumor suppressor CADM1 is involved in epithelial cell structure. Biochem. Biophys. Res. Commun. 2009, 390, 977–982. [Google Scholar] [CrossRef]
- Mimae, T.; Hagiyama, M.; Inoue, T.; Yoneshige, A.; Kato, T.; Okada, M.; Murakami, Y.; Ito, A. Increased ectodomain shedding of lung epithelial cell adhesion molecule 1 as a cause of increased alveolar cell apoptosis in emphysema. Thorax 2014, 69, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Hagiyama, M.; Yoneshige, A.; Inoue, T.; Sato, Y.; Mimae, T.; Okada, M.; Ito, A. The intracellular domain of cell adhesion molecule 1 is present in emphysematous lungs and induces lung epithelial cell apoptosis. J. Biomed. Sci. 2015, 22, 67. [Google Scholar] [CrossRef] [Green Version]
- Furuno, T.; Ito, A.; Koma, Y.; Watabe, K.; Yokozaki, H.; Bienenstock, J.; Nakanishi, M.; Kitamura, Y. The spermatogenic Ig superfamily/synaptic cell adhesion molecule mast-cell adhesion molecule promotes interaction with nerves. J. Immunol. 2005, 174, 6934–6942. [Google Scholar] [CrossRef] [Green Version]
- Hagiyama, M.; Nakatani, Y.; Takashima, Y.; Kato, T.; Inoue, T.; Kimura, R.; Otani, T.; Sato, Y.; Mori, H.; Arima, S.; et al. Urinary Cell Adhesion Molecule 1 Is a Novel Biomarker That Links Tubulointerstitial Damage to Glomerular Filtration Rates in Chronic Kidney Disease. Front. Cell Dev. Biol. 2019, 7, 111. [Google Scholar] [CrossRef]
- Koma, Y.I.; Furuno, T.; Hagiyama, M.; Hamaguchi, K.; Nakanishi, M.; Masuda, M.; Hirota, S.; Yokozaki, H.; Ito, A. Cell adhesion molecule 1 is a novel pancreatic-islet cell adhesion molecule that mediates nerve-islet cell interactions. Gastroenterology 2008, 134, 1544–1554. [Google Scholar] [CrossRef]
- Ito, A.; Nishikawa, Y.; Ohnuma, K.; Ohnuma, I.; Koma, Y.; Sato, A.; Enomoto, K.; Tsujimura, T.; Yokozaki, H. SgIGSF is a novel biliary-epithelial cell adhesion molecule mediating duct/ductule development. Hepatology 2007, 45, 684–694. [Google Scholar] [CrossRef] [Green Version]
- Stuber, J.C.; Kast, F.; Pluckthun, A. High-Throughput Quantification of Surface Protein Internalization and Degradation. ACS Chem. Biol. 2019, 14, 1154–1163. [Google Scholar] [CrossRef]
- Yasumi, M.; Shimizu, K.; Honda, T.; Takeuchi, M.; Takai, Y. Role of each immunoglobulin-like loop of nectin for its cell-cell adhesion activity. Biochem. Bioph. Res. Commun. 2003, 302, 61–66. [Google Scholar] [CrossRef]
- Ito, T.; Nakamura, A.; Tanaka, I.; Tsuboi, Y.; Morikawa, T.; Nakajima, J.; Takai, D.; Fukayama, M.; Sekido, Y.; Niki, T.; et al. CADM1 associates with Hippo pathway core kinases; membranous co-expression of CADM1 and LATS2 in lung tumors predicts good prognosis. Cancer Sci. 2019, 110, 2284–2295. [Google Scholar] [CrossRef]
- Reddy, P.; Deguchi, M.; Cheng, Y.; Hsueh, A.J. Actin cytoskeleton regulates Hippo signaling. PLoS ONE 2013, 8, e73763. [Google Scholar] [CrossRef]
- White, S.R.; Williams, P.; Wojcik, K.R.; Sun, S.; Hiemstra, P.S.; Rabe, K.F.; Dorscheid, D.R. Initiation of apoptosis by actin cytoskeletal derangement in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001, 24, 282–294. [Google Scholar] [CrossRef] [Green Version]
- Yageta, M.; Kuramochi, M.; Masuda, M.; Fukami, T.; Fukuhara, H.; Maruyama, T.; Shibuya, M.; Murakami, Y. Direct association of TSLC1 and DAL-1, two distinct tumor suppressor proteins in lung cancer. Cancer Res. 2002, 62, 5129–5133. [Google Scholar]
- Busam, R.D.; Thorsell, A.G.; Flores, A.; Hammarstrom, M.; Persson, C.; Obrink, B.; Hallberg, B.M. Structural Basis of Tumor Suppressor in Lung Cancer 1 (TSLC1) Binding to Differentially Expressed in Adenocarcinoma of the Lung (DAL-1/4.1B). J. Biol. Chem. 2011, 286, 4511–4516. [Google Scholar] [CrossRef] [Green Version]
- Moiseeva, E.P.; Straatman, K.R.; Leyland, M.L.; Bradding, P. CADM1 controls actin cytoskeleton assembly and regulates extracellular matrix adhesion in human mast cells. PLoS ONE 2014, 9, e85980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagiyama, M.; Ichiyanagi, N.; Kimura, K.B.; Muralkami, Y.; Ito, A. Expression of a Soluble Isoform of Cell Adhesion Molecule 1 in the Brain and Its Involvement in Directional Neurite Outgrowth. Am. J. Pathol. 2009, 174, 2278–2289. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Hagiyama, M.; Mimae, T.; Inoue, T.; Kato, T.; Yoneshige, A.; Nakanishi, J.; Kondo, T.; Okada, M.; Ito, A. alpha-Parvin, a pseudopodial constituent, promotes cell motility and is associated with lymph node metastasis of lobular breast carcinoma. Breast Cancer Res. Treat. 2014, 144, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Mimae, T.; Okada, M.; Hagiyama, M.; Miyata, Y.; Tsutani, Y.; Inoue, T.; Murakami, Y.; Ito, A. Upregulation of Notch2 and Six1 Is Associated with Progression of Early- Stage Lung Adenocarcinoma and a More Aggressive Phenotype at Advanced Stages. Clin. Cancer Res. 2012, 18, 945–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagiyama, M.; Kimura, R.; Yoneshige, A.; Inoue, T.; Otani, T.; Ito, A. Cell Adhesion Molecule 1 Contributes to Cell Survival in Crowded Epithelial Monolayers. Int. J. Mol. Sci. 2020, 21, 4123. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114123
Hagiyama M, Kimura R, Yoneshige A, Inoue T, Otani T, Ito A. Cell Adhesion Molecule 1 Contributes to Cell Survival in Crowded Epithelial Monolayers. International Journal of Molecular Sciences. 2020; 21(11):4123. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114123
Chicago/Turabian StyleHagiyama, Man, Ryuichiro Kimura, Azusa Yoneshige, Takao Inoue, Tomoyuki Otani, and Akihiko Ito. 2020. "Cell Adhesion Molecule 1 Contributes to Cell Survival in Crowded Epithelial Monolayers" International Journal of Molecular Sciences 21, no. 11: 4123. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21114123




