Pomegranate Mesocarp against Colitis-Induced Visceral Pain in Rats: Effects of a Decoction and Its Fractions
Abstract
:1. Introduction
2. Results
2.1. Composition of the Ellagitannins in Dry Decoction and in the Ellagitannin Fraction
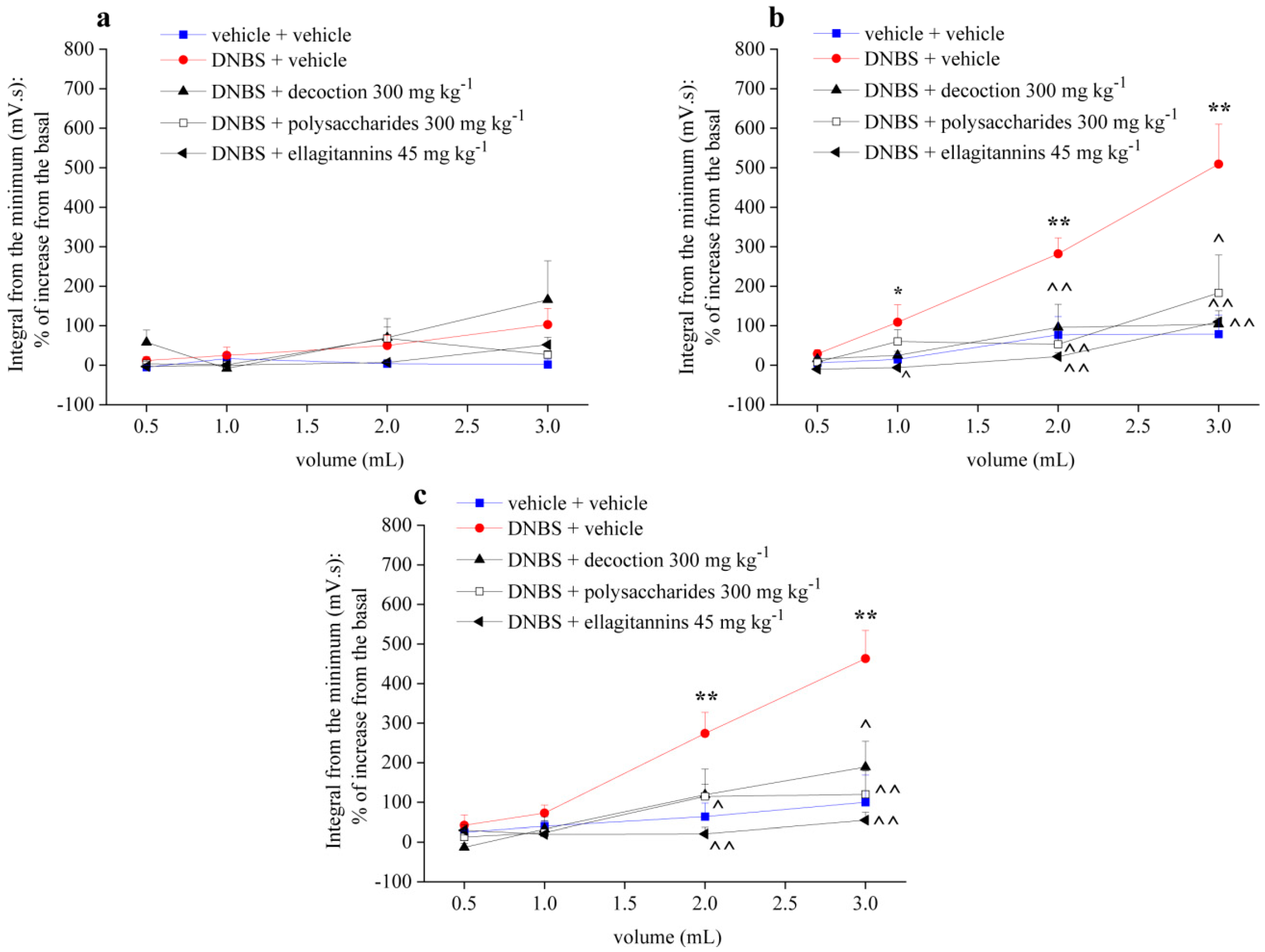
2.2. Effect of Repeated Treatment with Pomegranate-Based Preparations on Visceral Hypersensitivity
2.3. Effect of Repeated Treatment with Pomegranate-Based Preparations on Abdominal Withdrawal Response
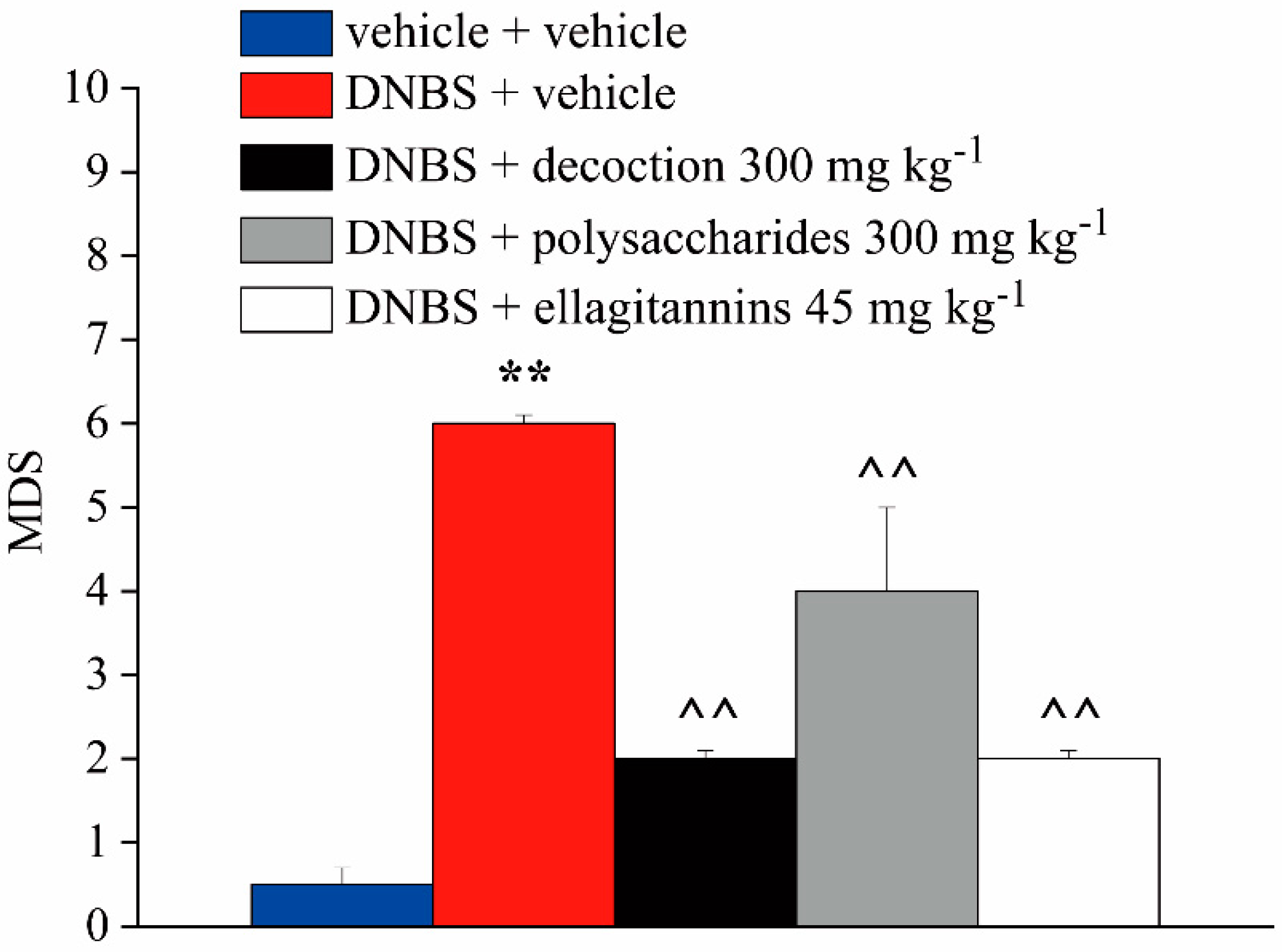
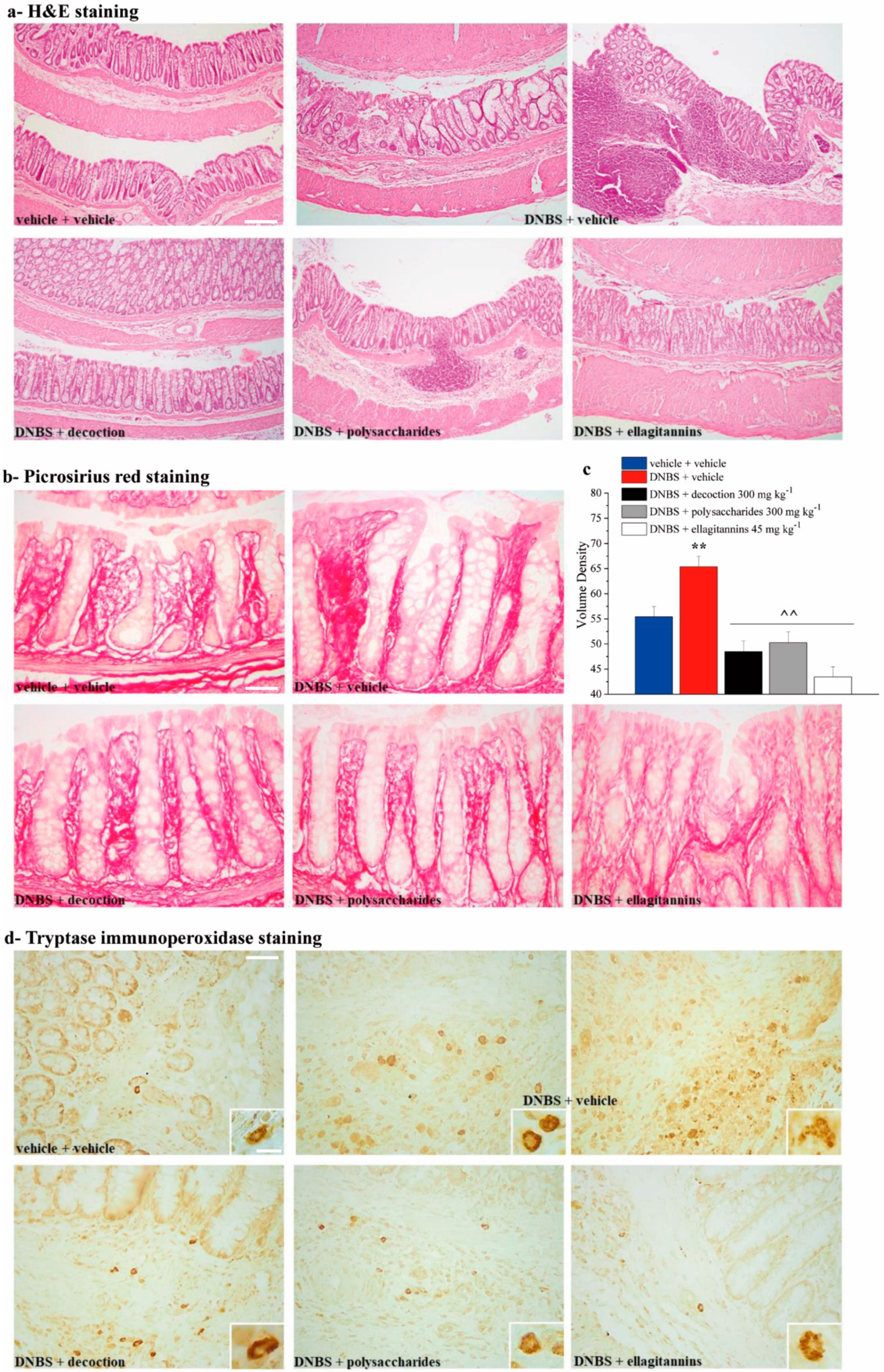
2.4. Effect of Repeated Treatment with Pomegranate-Based Preparations on Colon Damage
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Selection of the Tested Doses
4.3. Animals
4.4. Induction of Colitis
4.5. Drug Administrations
4.6. Assessment of Visceral Sensitivity by Visceromotor Response (VMR)
4.7. Assessment of Visceral Sensitivity by Abdominal Withdrawal Reflex (AWR)
4.8. Macroscopic and Microscopic Analysis of Tissue Damage
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AWR | Abdominal Withdrawal Reflex |
| CRD | Colon Rectal Distension |
| DNBS | 2,4-dinitrobenzenesulfonic acid |
| IBDs | Inflammatory Bowel Diseases |
| IBS | Irritable Bowel Syndrome |
| VMR | Visceromotor Response |
References
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A Review Study on Punica granatum L. J. Evid.-Based Complement. Altern. Med. 2016, 21, 221–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Said, F.A.; Opara, L.U.; Al-Yahyai, R.A. Physico-chemical and textural quality attributes of pomegranate cultivars (Punica granatum L.) grown in the Sultanate of Oman. J. Food Eng. 2009, 90, 129–134. [Google Scholar] [CrossRef]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Mahal, H.S.; Kapoor, S.; Aradhya, S.M. In Vitro Studies on the Binding, Antioxidant, and Cytotoxic Actions of Punicalagin. J. Agric. Food Chem. 2007, 55, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary Metabolites, Anthocyanins, and Hydrolyzable Tannins in the Pomegranate Fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate Juice Ellagitannin Metabolites Are Present in Human Plasma and Some Persist in Urine for Up to 48 Hours. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef] [Green Version]
- Taghi Mansouri, M.; Naghizadeh, B.; Ghorbanzadeh, B.; Farbood, Y. Central and peripheral antinociceptive effects of ellagic acid in different animal models of pain. Eur. J. Pharmacol. 2013, 707, 46–53. [Google Scholar] [CrossRef]
- Gainok, J.; Daniels, R.; Golembiowski, D.; Kindred, P.; Post, L.; Strickland, R.; Garrett, N. Investigation of the anti-inflammatory, antinociceptive effect of ellagic acid as measured by digital paw pressure via the Randall-Selitto meter in male Sprague-Dawley rats. AANA J. 2011, 79, S28–S34. [Google Scholar]
- Rogerio, A.P.; Fontanari, C.; Melo, M.C.C.; Ambrosio, S.R.; de Souza, G.E.P.; Pereira, P.S.; França, S.C.; da Costa, F.B.; Albuquerque, D.A.; Faccioli, L.H. Anti-inflammatory, analgesic and anti-oedematous effects of Lafoensia pacari extract and ellagic acid. J. Pharm. Pharmacol. 2006, 58, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Zeghad, N.; Ahmed, E.; Belkhiri, A.; Heyden, Y.V.; Demeyer, K. Antioxidant activity of Vitis vinifera, Punica granatum, Citrus aurantium and Opuntia ficus indica fruits cultivated in Algeria. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rios-Corripio, G.; Guerrero-Beltrán, J.Á. Antioxidant and physicochemical characteristics of unfermented and fermented pomegranate (Punica granatum L.) beverages. J. Food Sci. Technol. 2019, 56, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.; Patel, P.; Kothari, V. Anti-infective potential of hydroalcoholic extract of Punica granatum peel against gram-negative bacterial pathogens. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, N.; Jamil, A.; Shah, S.W.A.; Shah, I.; Ahmed, G. Spasmogenic and spasmolytic activity of rind of Punica granatum Linn. BMC Complement. Altern. Med. 2017, 17. [Google Scholar] [CrossRef] [Green Version]
- Actis, G.C.; Pellicano, R.; Fagoonee, S.; Ribaldone, D.G. History of Inflammatory Bowel Diseases. J. Clin. Med. 2019, 8, 1970. [Google Scholar] [CrossRef] [Green Version]
- Adriani, A.; Ribaldone, D.G.; Astegiano, M.; Durazzo, M.; Saracco, G.M.; Pellicano, R. Irritable bowel syndrome: The clinical approach. Panminerva Med. 2018, 60. [Google Scholar] [CrossRef]
- Coward, S.; Clement, F.; Benchimol, E.I.; Bernstein, C.N.; Avina-Zubieta, J.A.; Bitton, A.; Carroll, M.W.; Hazlewood, G.; Jacobson, K.; Jelinski, S.; et al. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology 2019, 156, 1345–1353.e4. [Google Scholar] [CrossRef] [Green Version]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Furey, T.S.; Sethupathy, P.; Sheikh, S.Z. Redefining the IBDs using genome-scale molecular phenotyping. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 296–311. [Google Scholar] [CrossRef]
- Vahedi, H.; Ansari, R.; Mir-Nasseri, M.; Jafari, E. Irritable Bowel Syndrome: A Review Article. Middle East J. Dig. Dis. 2010, 2, 66–77. [Google Scholar]
- Spiller, R.; Major, G. IBS and IBD—Separate entities or on a spectrum? Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Srinath, A.I.; Walter, C.; Newara, M.C.; Szigethy, E.M. Pain management in patients with inflammatory bowel disease: Insights for the clinician. Ther. Adv. Gastroenterol. 2012, 5, 339–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Boeckxstaens, G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef]
- Sanchez-Muñoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. WJG 2008, 14, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Rosso, C.; Stalla, F.; Rizzo, M.; Massano, A.; Abate, M.L.; Olivero, A.; Armandi, A.; Vanni, E.; Younes, R.; et al. On-Treatment Decrease of Serum Interleukin-6 as a Predictor of Clinical Response to Biologic Therapy in Patients with Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- He, S.-H. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 309. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X.; Zhao, J.; Yang, S.; Dong, L.; Qin, B. GPER-mediated, oestrogen-dependent visceral hypersensitivity in stressed rats is associated with mast cell tryptase and histamine expression. Fundam. Clin. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Frei, S.M.; Stevens, R.L. The multifaceted mast cell in inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 2364–2378. [Google Scholar] [CrossRef] [Green Version]
- Governa, P.; Marchi, M.; Cocetta, V.; De Leo, B.; Saunders, P.T.K.; Catanzaro, D.; Miraldi, E.; Montopoli, M.; Biagi, M. Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an In Vitro Intestinal Inflammation Model Using Immune Cells and Caco-2. Pharm. Basel Switz. 2018, 11, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Vindigni, S.M.; Zisman, T.L.; Suskind, D.L.; Damman, C.J. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: A tripartite pathophysiological circuit with implications for new therapeutic directions. Ther. Adv. Gastroenterol. 2016, 9, 606–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, E.; Parisio, C.; Branca, J.J.V.; Segnani, C.; Ippolito, C.; Pellegrini, C.; Antonioli, L.; Fornai, M.; Micheli, L.; Pacini, A.; et al. Deepening the mechanisms of post-inflammatory visceral pain persistence: An evaluation of the gut-spinal cord relationship. Cells 2016. under review. [Google Scholar]
- Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Di Cesare Mannelli, L.; Antonini, G.; Panizzi, E.; Maidecchi, A.; Giovagnoni, E.; Lucci, J.; et al. Researching New Therapeutic Approaches for Abdominal Visceral Pain Treatment: Preclinical Effects of an Assembled System of Molecules of Vegetal Origin. Nutrients 2019, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Morampudi, V.; Bhinder, G.; Wu, X.; Dai, C.; Sham, H.P.; Vallance, B.A.; Jacobson, K. DNBS/TNBS Colitis Models: Providing Insights Into Inflammatory Bowel Disease and Effects of Dietary Fat. J. Vis. Exp. JoVE 2014. [Google Scholar] [CrossRef] [Green Version]
- Gschossmann, J.M.; Liebregts, T.; Adam, B.; Buenger, L.; Ruwe, M.; Gerken, G.; Holtmann, G. Long-Term Effects of Transient Chemically Induced Colitis on the Visceromotor Response to Mechanical Colorectal Distension. Dig. Dis. Sci. 2004, 49, 96–101. [Google Scholar] [CrossRef]
- Adam, B.; Liebregts, T.; Best, J.; Bechmann, L.; Lackner, C.; Neumann, J.; Koehler, S.; Holtmann, G. A combination of peppermint oil and caraway oil attenuates the post-inflammatory visceral hyperalgesia in a rat model. Scand. J. Gastroenterol. 2006, 41, 155–160. [Google Scholar] [CrossRef]
- Hughes, P.A.; Brierley, S.M.; Blackshaw, L.A. Post-inflammatory modification of colonic afferent mechanosensitivity. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1034–1040. [Google Scholar] [CrossRef]
- Ghia, J.-E.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El-Sharkawy, R.T.; Côté, F.; Mallet, J.; Khan, W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 2009, 137, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Martín, R.; Chain, F.; Chain, F.; Miquel, S.; Miquel, S.; Natividad, J.M.; Natividad, J.M.; Sokol, H.; Sokol, H.; et al. Effects in the use of a genetically engineered strain of Lactococcus lactis delivering in situ IL-10 as a therapy to treat low-grade colon inflammation. Hum. Vaccines Immunother. 2014, 10, 1611–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielińska, A.; Sałaga, M.; Włodarczyk, M.; Fichna, J. Focus on current and future management possibilities in inflammatory bowel disease-related chronic pain. Int. J. Colorectal Dis. 2019, 34, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassaganya-Riera, J.; Reynolds, K.; Martino-Catt, S.; Cui, Y.; Hennighausen, L.; Gonzalez, F.; Rohrer, J.; Benninghoff, A.U.; Hontecillas, R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 2004, 127, 777–791. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Bird, H.; Giannoudis, P.V. Nonsteroidal Anti-inflammatory Drugs: Prostaglandins, Indications, and Side Effects. Available online: https://www.dovepress.com/nonsteroidal-anti-inflammatory-drugs-prostaglandins-indications-and-si-peer-reviewed-article-IJICMR (accessed on 24 March 2020).
- Pillai, N.; Dusheiko, M.; Burnand, B.; Pittet, V. A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS ONE 2017, 12, e0185500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, Gallotannins and their Metabolites—The Contribution to the Anti-Inflammatory Effect of Food Products and Medicinal Plants. Curr. Med. Chem. 2018, 25, 4946–4967. [Google Scholar] [CrossRef]
- Russel, M.G.; Engels, L.G.; Muris, J.W.; Limonard, C.B.; Volovics, A.; Brummer, R.J.; Stockbrügger, R.W. Modern life’ in the epidemiology of inflammatory bowel disease: A case-control study with special emphasis on nutritional factors. Eur. J. Gastroenterol. Hepatol. 1998, 10, 243–249. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases-Promises, Perspectives, and Pitfalls. Oxid. Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef] [Green Version]
- Sigall-Boneh, R.; Levine, A.; Lomer, M.; Wierdsma, N.; Allan, P.; Fiorino, G.; Gatti, S.; Jonkers, D.; Kierkus, J.; Katsanos, K.H.; et al. Research Gaps in Diet and Nutrition in Inflammatory Bowel Disease. A Topical Review by D-ECCO Working Group [Dietitians of ECCO]. J. Crohns Colitis 2017, 11, 1407–1419. [Google Scholar] [CrossRef]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent health effects of pomegranate. Adv. Biomed. Res. 2014, 3. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Mol. Basel Switz. 2017, 22, 1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeram, N.; Lee, R.; Hardy, M.; Heber, D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005, 41, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Jurenka, J.S. Therapeutic applications of pomegranate (Punica granatum L.): A review. Altern. Med. Rev. J. Clin. Ther. 2008, 13, 128–144. [Google Scholar]
- Nuamsetti, T.; Dechayuenyong, P.; Tantipaibulvut, S. Antibacterial activity of pomegranate fruit peels and arils. ScienceAsia 2012, 38, 319. [Google Scholar] [CrossRef] [Green Version]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Valadares, M.C.; Pereira, E.R.T.; Benfica, P.L.; Paula, J.R. Assessment of mutagenic and antimutagenic effects of Punica granatum in mice. Braz. J. Pharm. Sci. 2010, 46, 121–127. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X. Optimization of extraction process of crude polysaccharides from Pomegranate peel by response surface methodology. Carbohydr. Polym. 2013, 92, 1197–1202. [Google Scholar] [CrossRef]
- Rout, S.; Banerjee, R. Free radical scavenging, anti-glycation and tyrosinase inhibition properties of a polysaccharide fraction isolated from the rind from Punica granatum. Bioresour. Technol. 2007, 98, 3159–3163. [Google Scholar] [CrossRef]
- Joseph, M.M.; Aravind, S.R.; Varghese, S.; Mini, S.; Sreelekha, T.T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012, 5, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Joseph, M.M.; Aravind, S.R.; George, S.K.; Varghese, S.; Sreelekha, T.T. A galactomannan polysaccharide from Punica granatum imparts in vitro and in vivo anticancer activity. Carbohydr. Polym. 2013, 98, 1466–1475. [Google Scholar] [CrossRef]
- Sorrenti, V.; Randazzo, C.L.; Caggia, C.; Ballistreri, G.; Romeo, F.V.; Fabroni, S.; Timpanaro, N.; Raffaele, M.; Vanella, L. Beneficial Effects of Pomegranate Peel Extract and Probiotics on Pre-adipocyte Differentiation. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wu, S.; Li, Z.; Li, J.; Li, X.; Xiang, J.; Ding, H. Wild jujube polysaccharides protect against experimental inflammatory bowel disease by enabling enhanced intestinal barrier function. Food Funct. 2015, 6, 2568–2577. [Google Scholar] [CrossRef]
- Hung, T.V.; Suzuki, T. Dietary Fermentable Fiber Reduces Intestinal Barrier Defects and Inflammation in Colitic Mice. J. Nutr. 2016, 146, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Segarra, S.; Martínez-Subiela, S.; Cerdà-Cuéllar, M.; Martínez-Puig, D.; Muñoz-Prieto, A.; Rodríguez-Franco, F.; Rodríguez-Bertos, A.; Allenspach, K.; Velasco, A.; Cerón, J. Oral chondroitin sulfate and prebiotics for the treatment of canine Inflammatory Bowel Disease: A randomized, controlled clinical trial. BMC Vet. Res. 2016, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.N.; So, H.L.; Liu, E.S.L.; Shin, V.Y.; Cho, C.H. Effect of polysaccharides from Angelica sinensis on gastric ulcer healing. Life Sci. 2003, 72, 925–932. [Google Scholar] [CrossRef]
- Maria-Ferreira, D.; da Silva, L.M.; Mendes, D.A.G.B.; de Cabrini, D.A.; Nascimento, A.M.; Iacomini, M.; Cipriani, T.R.; Santos, A.R.S.; de Werner, M.F.P.; Baggio, C.H. Rhamnogalacturonan from Acmella oleracea (L.) R.K. Jansen: Gastroprotective and ulcer healing properties in rats. PLoS ONE 2014, 9, e84762. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64 (Suppl. 3), S112–S120. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Bergmann, H.; Rogoll, D.; Scheppach, W.; Melcher, R.; Richling, E. The Ussing type chamber model to study the intestinal transport and modulation of specific tight-junction genes using a colonic cell line. Mol. Nutr. Food Res. 2009, 53, 1211–1225. [Google Scholar] [CrossRef]
- Amasheh, M.; Andres, S.; Amasheh, S.; Fromm, M.; Schulzke, J.-D. Barrier effects of nutritional factors. Ann. N. Y. Acad. Sci. 2009, 1165, 267–273. [Google Scholar] [CrossRef]
- Rogoll, D.; Bergmann, H.F.; Hellenschmidt, D.; Heinze, J.; Scheppach, W.; Melcher, R.; Richling, E. Influence of Apple Polyphenols on the Intestinal Barrier in a Colonic Cell Model. Available online: /paper/Influence-of-apple-polyphenols-on-the-intestinal-in-Rogoll-Bergmann/0c0dcbda57661f08afe317b1afe1444be497641f (accessed on 24 March 2020).
- Carrasco-Pozo, C.; Morales, P.; Gotteland, M. Polyphenols protect the epithelial barrier function of Caco-2 cells exposed to indomethacin through the modulation of occludin and zonula occludens-1 expression. J. Agric. Food Chem. 2013, 61, 5291–5297. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.T.; Zhang, Y.J.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, F.A.; Espín, J.C. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef]
- Kumar, S.; Votta, B.J.; Rieman, D.J.; Badger, A.M.; Gowen, M.; Lee, J.C. IL-1- and TNF-induced bone resorption is mediated by p38 mitogen activated protein kinase. J. Cell. Physiol. 2001, 187, 294–303. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Larrosa, M.; Tomás-Barberán, F.A.; Dolara, P.; Espín, J.C. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br. J. Nutr. 2010, 104, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far. Available online: https://www.hindawi.com/journals/ecam/2013/270418/ (accessed on 9 June 2020).
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krammer, L.; Sowa, A.S.; Lorentz, A. Mast Cells in Irritable Bowel Syndrome: A Systematic Review. J. Gastrointest. Liver Dis. JGLD 2019, 28, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.; Giuliani, C.; Rossi, F.; Adessi, A.; Al-Tamimi, A.; Mazzola, G.; Di Gioia, D.; Innocenti, M.; Mulinacci, N. Polysaccharides from by-products of the Wonderful and Laffan pomegranate varieties: New insight into extraction and characterization. Food Chem. 2017, 235, 58–66. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [Green Version]
- Fornai, M.; Blandizzi, C.; Antonioli, L.; Colucci, R.; Bernardini, N.; Segnani, C.; De Ponti, F.; Del Tacca, M. Differential role of cyclooxygenase 1 and 2 isoforms in the modulation of colonic neuromuscular function in experimental inflammation. J. Pharmacol. Exp. Ther. 2006, 317, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.A.; Gebhart, G.F. Assessment of colon sensitivity by luminal distension in mice. Nat. Protoc. 2007, 2, 2624–2631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, C.; Tang, Y.; Chen, A.-Q.; Liu, C.-Y.; Lu, D.-L. ZD 7288, an HCN channel blocker, attenuates chronic visceral pain in irritable bowel syndrome-like rats. World J. Gastroenterol. WJG 2014, 20, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Fornai, M.; Colucci, R.; Ghisu, N.; Da Settimo, F.; Natale, G.; Kastsiuchenka, O.; Duranti, E.; Virdis, A.; Vassalle, C.; et al. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J. Pharmacol. Exp. Ther. 2007, 322, 435–442. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parisio, C.; Lucarini, E.; Micheli, L.; Toti, A.; Khatib, M.; Mulinacci, N.; Calosi, L.; Bani, D.; Di Cesare Mannelli, L.; Ghelardini, C. Pomegranate Mesocarp against Colitis-Induced Visceral Pain in Rats: Effects of a Decoction and Its Fractions. Int. J. Mol. Sci. 2020, 21, 4304. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124304
Parisio C, Lucarini E, Micheli L, Toti A, Khatib M, Mulinacci N, Calosi L, Bani D, Di Cesare Mannelli L, Ghelardini C. Pomegranate Mesocarp against Colitis-Induced Visceral Pain in Rats: Effects of a Decoction and Its Fractions. International Journal of Molecular Sciences. 2020; 21(12):4304. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124304
Chicago/Turabian StyleParisio, Carmen, Elena Lucarini, Laura Micheli, Alessandra Toti, Mohamad Khatib, Nadia Mulinacci, Laura Calosi, Daniele Bani, Lorenzo Di Cesare Mannelli, and Carla Ghelardini. 2020. "Pomegranate Mesocarp against Colitis-Induced Visceral Pain in Rats: Effects of a Decoction and Its Fractions" International Journal of Molecular Sciences 21, no. 12: 4304. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21124304






