Electronic-Cigarette Vehicles and Flavoring Affect Lung Function and Immune Responses in a Murine Model
Abstract
:1. Introduction
2. Results
2.1. VG/PG Plus Vanilla Impaired Lung Functional Parameters
2.2. VG/PG and VG/PG Plus Vanilla Did not Alter Lung Macrophage Counts
2.3. VG/PG Altered Lung Cell Immunophenotype
2.4. VG/PG Increased Levels of Lipid Mediators
2.5. Alteration of Gene Expression by VG/PG and VG/PG plus Vanilla
2.6. Immunoglobulin Levels Were Altered by VG/PG plus Vanilla
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Mice
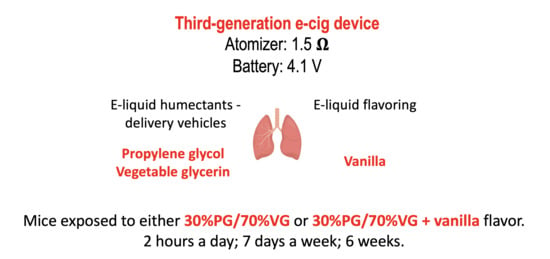
4.3. E-Cig Aerosol Exposures
4.4. Pulmonary Function Testing
4.5. Tissue Staining
4.6. Lung Immunophenotype by Flow Cytometry
4.7. Extraction of Lipid Mediators
4.8. Lung mRNA Extraction and Gene Expression Analysis by Quantitative RT-PCR
4.9. RT2 Profiler PCR Array
4.10. Immunoglobulin Level Determination by ELISA
4.11. Protein Analysis for ELISA and Lipid Mediator Extraction Standardization
4.12. Ingenuity Pathway Analysis (IPA)
4.13. Statistical Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 12-HETE | 12-hydroxyeicosatetraenoic acid |
| 2AG | 2-arachidonoylglycerol |
| AA | arachidonic acid |
| AM | alveolar macrophages |
| BALF | broncho-alveolar lavage fluid |
| CD4 | cluster of differentiation 4 |
| CD8 | cluster of differentiation 8 |
| CD19 | cluster of differentiation 19 |
| CDC | Center for Disease Control and Prevention |
| DC | dendritic cells |
| e-cig | electronic-cigarette |
| COPD | chronic obstructive pulmonary disease |
| ENDS | electronic-nicotine delivery systems |
| EVALI | e-cigarette or vaping product use-associated acute lung injury |
| FDA | Food and Drug Administration |
| GRAS | generally recognized as safe |
| IgG | immunoglobulin G |
| IM | interstitial macrophages |
| MAO | monoamine oxidase |
| NEU | neutrophils |
| NIOSH | National Institute of Occupational Safety and Health |
| NK | natural killer cells |
| OEA | oleoylethanolamide |
| PEA | palmitoylethanolamide |
| PGD2 | prostaglandin D2 |
| PGE2 | prostaglandin E2 |
| PG | propylene glycol |
| VG | vegetable glycerin |
| SP-A-D | surfactant protein A-D |
| va | vanilla |
| THC | tetrahydrocannabinol |
References
- CDC. Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products 2019. Available online: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed on 25 November 2019).
- Lewis, N.; McCaffrey, K.; Sage, K.; Cheng, C.J.; Green, J.; Goldstein, L.; Campbell, H.; Ferrell, D.; Malan, N.; LaCross, N.; et al. E-cigarette Use, or Vaping, Practices and Characteristics Among Persons with Associated Lung Injury-Utah, April-October 2019. MMWR Morb Mortal Wkly Rep. 2019, 68, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Sommerfeld, C.G.; Weiner, D.J.; Nowalk, A.; Larkin, A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome from E-Cigarette Use. Pediatrics 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Aoshiba, K.; Herai, Y.; Nakamura, H.; Takemura, T. Lung injury associated with electronic cigarettes inhalation diagnosed by transbronchial lung biopsy. Respirol. Case Rep. 2018, 6, e00282. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Sangani, R.; Alhajhusain, A. Acute Lipoid Pneumonia Secondary to E-Cigarettes Use: An Unlikely Replacement for Cigarettes. CHEST J. 2015, 148, 382A. [Google Scholar] [CrossRef]
- Arter, Z.L.; Wiggins, A.; Hudspath, C.; Kisling, A.; Hostler, D.C.; Hostler, J.M. Acute eosinophilic pneumonia following electronic cigarette use. Respir. Med. Case Rep. 2019, 27, 100825. [Google Scholar] [CrossRef]
- Balmes, J.R. Reply to: Are Electronic Cigarette Users at Risk for Lipid-Mediated Lung Injury? Am. J. Respir. Crit Care Med. 2020. [Google Scholar] [CrossRef]
- Chun, L.F.; Moazed, F.; Calfee, C.S.; Matthay, M.A.; Gotts, J.E. Pulmonary toxicity of e-cigarettes. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 313, L193–L206. [Google Scholar] [CrossRef]
- Madison, M.C.; Landers, C.T.; Gu, B.H.; Chang, C.Y.; Tung, H.Y.; You, R.; Hong, M.J.; Baghaei, N.; Song, L.-Z.; Porter, P.; et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019, 129, 4290–4304. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, B.H.; Butkera, J.P. Outbreak of Severe Pulmonary Disease Linked to E-Cigarette Use: The Makings of a Mass Tort? Mass Torts 2020, 18, 8–12. [Google Scholar]
- Chen-Sankey, J.C.; Kong, G.; Choi, K. Perceived ease of flavored e-cigarette use and e-cigarette use progression among youth never tobacco users. PLoS ONE 2019, 14, e0212353. [Google Scholar] [CrossRef]
- Fadus, M.C.; Smith, T.T.; Squeglia, L.M. The rise of e-cigarettes, pod mod devices, and JUUL among youth: Factors influencing use, health implications, and downstream effects. Drug Alcohol Depend. 2019, 201, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Husari, A.; Shihadeh, A.; Talih, S.; Hashem, Y.; El Sabban, M.; Zaatari, G. Acute Exposure to Electronic and Combustible Cigarette Aerosols: Effects in an Animal Model and in Human Alveolar Cells. Nicotine Tob. Res. 2016, 18, 613–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef] [PubMed]
- Glynos, C.; Bibli, S.I.; Katsaounou, P.; Pavlidou, A.; Magkou, C.; Karavana, V.; Topouzis, S.; Kalomenidis, I.; Zakynthinos, S.; Papapetropoulos, A. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L662–L672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, A.; Lugg, S.T.; Aldridge, K.; Lewis, K.E.; Bowden, A.; Mahida, R.Y.; Grudzinska, F.S.; Dosanjh, D.; Parekh, D.; Foronjy, R.; et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 2018, 73, 1161–1169. [Google Scholar] [CrossRef] [Green Version]
- Alexander, L.E.C.; Drummond, C.A.; Hepokoski, M.; Mathew, D.P.; Moshensky, A.; Willeford, A.; Das, S.; Singh, P.; Yong, Z.; Lee, J.H.; et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R834–R847. [Google Scholar] [CrossRef] [Green Version]
- Chapman, D.G.; Casey, D.T.; Ather, J.L.; Aliyeva, M.; Daphtary, N.; Lahue, K.G.; Van Der Velden, J.L.; Janssen-Heininger, Y.M.W.; Irvin, C.G. The Effect of Flavored E-cigarettes on Murine Allergic Airways Disease. Sci. Rep. 2019, 9, 13671. [Google Scholar] [CrossRef]
- Cardenia, V.; Vivarelli, F.; Cirillo, S.; Paolini, M.; Canistro, D.; Rodriguez-Estrada, M.T. The effect of electronic-cigarettes aerosol on rat brain lipid profile. Biochimie 2018, 153, 99–108. [Google Scholar] [CrossRef]
- Singanayagam, A.; Snelgrove, R.J. Less burn, more fat: Electronic cigarettes and pulmonary lipid homeostasis. J. Clin. Investig. 2019, 129, 4077–4079. [Google Scholar] [CrossRef] [Green Version]
- Maddock, S.D.; Cirulis, M.M.; Callahan, S.J.; Keenan, L.M.; Pirozzi, C.S.; Raman, S.M.; Aberegg, S.K. Pulmonary Lipid-Laden Macrophages and Vaping. N. Engl. J. Med. 2019, 381, 1488–1489. [Google Scholar] [CrossRef]
- Kooragayalu, S.; El-Zarif, S.; Jariwala, S. Vaping associated pulmonary injury (VAPI) with superimposed mycoplasma pneumoniae infection. Respir. Med. Case Rep. 2020, 29, 1000997. [Google Scholar] [CrossRef] [PubMed]
- Weaver, T.E.; Whitsett, J.A. Function and regulation of expression of pulmonary surfactant-associated proteins. Biochem. J. 1991, 273, 249–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Przybyla, R.J.; Wright, J.; Parthiban, R.; Nazemidashtarjandi, S.; Kaya, S.; Farnoud, A.M. Electronic cigarette vapor alters the lateral structure but not tensiometric properties of calf lung surfactant. Respir. Res. 2017, 18, 193. [Google Scholar] [CrossRef] [PubMed]
- Sosnowski, T.R.; Jabłczyńska, K.; Odziomek, M.; Schlage, W.K.; Kuczaj, A.K. Physicochemical studies of direct interactions between lung surfactant and components of electronic cigarettes liquid mixtures. Inhal. Toxicol. 2018, 30, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, C.; McKeganey, N.; Dickson, T.; Nides, M. Changing patterns of first e-cigarette flavor used and current flavors used by 20,836 adult frequent e-cigarette users in the USA. Harm Reduct. J. 2018, 15, 33. [Google Scholar] [CrossRef] [Green Version]
- Erythropel, H.C.; Jabba, S.V.; DeWinter, T.M.; Mendizabal, M.; Anastas, P.T.; Jordt, S.-E.; Zimmerman, J. Formation of flavorant–propylene Glycol Adducts With Novel Toxicological Properties in Chemically Unstable E-Cigarette Liquids. Nicotine Tob. Res. 2018, 21, 1248–1258. [Google Scholar] [CrossRef]
- Truman, P.; Stanfill, S.; Heydari, A.; Silver, E.; Fowles, J. Monoamine oxidase inhibitory activity of flavoured e-cigarette liquids. Neurotoxicology 2019, 75, 123–128. [Google Scholar] [CrossRef]
- Sleiman, M.; Logue, J.M.; Montesinos, V.N.; Russell, M.L.; Litter, M.I.; Gundel, L.A.; Destaillats, H. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ. Sci. Technol. 2016, 50, 9644–9651. [Google Scholar] [CrossRef] [Green Version]
- Geiss, O.; Bianchi, I.; Barrero-Moreno, J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 2016, 219, 268–277. [Google Scholar] [CrossRef]
- Prochaska, J.J. The public health consequences of e-cigarettes: A review by the National Academies of Sciences. A call for more research, a need for regulatory action. Addiction 2019, 114, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Wieslander, G.; Norbäck, D.; Lindgren, T. Experimental exposure to propylene glycol mist in aviation emergency training: Acute ocular and respiratory effects. Occup Environ. Med. 2001, 58, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Schmidbauer, N.; Spengler, J.; Bornehag, C.-G. Sources of propylene glycol and glycol ethers in air at home. Int. J. Environ. Res. Public Health 2010, 7, 4213–4237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erythropel, H.C.; Davis, L.M.; de Winter, T.M.; Jordt, S.E.; Anastas, P.T.; O’Malley, S.S.; Krishnan-Sarin, S.; Zimmerman, J. Flavorant-Solvent Reaction Products and Menthol in JUUL E-Cigarettes and Aerosol. Am. J. Prev. Med. 2019, 57, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.-Z.; Li, G.-M.; Tian, Y.; Song, B.; Shi, R. Decreased expression of FOXF2 as new predictor of poor prognosis in stage I non-small cell lung cancer. Oncotarget 2016, 7, 55601–55610. [Google Scholar] [CrossRef] [PubMed]
- Doumanov, J.; Jordanova, A.; Zlatkov, K.; Moskova-Doumanova, V.; Lalchev, Z. Investigation of IL-6 Effects on SP-A Expression in A549 Lung Cell Line. Biotechnol. Biotechnol. Equip. 2012, 26, 96–99. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, J.; Grijalva, V.; Hama, S.; Barbour, K.; Berger, F.G.; Navab, M.; Fogelman, A.M.; Reddy, S.T. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J. Biol. Chem. 2009, 284, 18292–18301. [Google Scholar] [CrossRef] [Green Version]
- Canistro, D.; Vivarelli, F.; Cirillo, S.; Marquillas, C.B.; Buschini, A.; Lazzaretti, M.; Marchi, L.; Cardenia, V.; Rodriguez-Estrada, M.T.; Lodovici, M.; et al. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep. 2017, 7, 2028. [Google Scholar] [CrossRef]
- Irvin, C.G.; Bates, J.H.T. Measuring the lung function in the mouse: The challenge of size. Respir. Res. 2003, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Bachofen, H.; Scherrer, M. Lung tissue resistance in diffuse interstitial pulmonary fibrosis. J. Clin. Investig. 1967, 46, 133–140. [Google Scholar] [CrossRef]
- Ström, J.E.; Pourazar, J.; Linder, R.; Blomberg, A.; Lindberg, A.; Bucht, A.; Behndig, A.F. Cytotoxic lymphocytes in COPD airways: Increased NK cells associated with disease, iNKT and NKT-like cells with current smoking. Respir. Res. 2018, 19, 244. [Google Scholar] [CrossRef] [Green Version]
- Hansen, M.J.; Chan, S.P.J.; Langenbach, S.Y.; Dousha, L.F.; Jones, J.E.; Yatmaz, S.; Seow, H.J.; Vlahos, R.; Anderson, G.P.; Bozinovski, S. IL-17A and serum amyloid A are elevated in a cigarette smoke cessation model associated with the persistence of pigmented macrophages, neutrophils and activated NK cells. PLoS ONE 2014, 9, e113180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motz, G.T.; Eppert, B.L.; Wortham, B.W.; Amos-Kroohs, R.M.; Flury, J.L.; Wesselkamper, S.C.; Borchers, M.T. Chronic cigarette smoke exposure primes NK cell activation in a mouse model of chronic obstructive pulmonary disease. J. Immunol. 2010, 184, 4460–4469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, J.S.; Logan, J.; Wang, G.-J.; Volkow, N.D.; Telang, F.; Zhu, W.; Franceschi, D.; Shea, C.; Garza, V.; Xu, Y.; et al. Comparison of monoamine oxidase a in peripheral organs in nonsmokers and smokers. J. Nucl. Med. 2005, 46, 1414–1420. [Google Scholar] [PubMed]
- Andreas, S.; Haarmann, H.; Klarner, S.; Hasenfuß, G.; Raupach, T. Increased sympathetic nerve activity in COPD is associated with morbidity and mortality. Lung 2013, 192, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Joho, S.; Ushijima, R.; Akabane, T.; Hirai, T.; Inoue, H. Restrictive Lung Function Is Related to Sympathetic Hyperactivity in Patients With Heart Failure. J. Card. Fail. 2017, 23, 96–103. [Google Scholar] [CrossRef]
- Collins, R.A.; Ikegami, M.; Korfhagen, T.R.; Whitsett, J.A.; Sly, P.D. In Vivo measurements of changes in respiratory mechanics with age in mice deficient in surfactant protein D. Pediatr. Res. 2003, 53, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Manali, E.D.; Moschos, C.; Triantafillidou, C.; Kotanidou, A.; Psallidas, I.; Karabela, S.P.; Roussos, C.; Papiris, S.A.; Armaganidis, A.; Stathopoulos, G.T.; et al. Static and dynamic mechanics of the murine lung after intratracheal bleomycin. BMC Pulm. Med. 2011, 11, 33. [Google Scholar] [CrossRef]
- Darrah, R.J.; Mitchell, A.L.; Campanaro, C.K.; Barbato, E.S.; Litman, P.; Sattar, A.; Hodges, C.A.; Drumm, M.L.; Jacono, F.J. Early pulmonary disease manifestations in cystic fibrosis mice. J. Cyst. Fibros. 2016, 15, 736–744. [Google Scholar] [CrossRef]
- McGovern, T.K.; Robichaud, A.; Fereydoonzad, L.; Schuessler, T.F.; Martin, J.G. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J. Vis. Exp. 2013, 75, e50172. [Google Scholar] [CrossRef]
- Ferguson, G.T. Why does the lung hyperinflate? Proc. Am. Thorac. Soc. 2006, 3, 176–179. [Google Scholar] [CrossRef]
- Soffler, M.I.; Hayes, M.M.; Schwartzstein, R.M. Respiratory Sensations in Dynamic Hyperinflation: Physiological and Clinical Applications. Respir. Care 2017, 62, 1212–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saey, D.; Gagnon, P.; Guenette, J.A.; Langer, D.; LaViolette, L.; Mainguy, V.; Maltais, F.; Ribeiro, F. Pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 187–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larcombe, A.N.; Janka, M.A.; Mullins, B.; Berry, L.J.; Bredin, A.; Franklin, P.J. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, L67–L79. [Google Scholar] [CrossRef] [PubMed]
- Vardavas, C.I.; Anagnostopoulos, N.; Kougias, M.; Evangelopoulou, V.; Connolly, G.N.; Behrakis, P.K. Short-term pulmonary effects of using an electronic cigarette: Impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 2012, 141, 1400–1406. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.D.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2015, 25, e10–e15. [Google Scholar] [CrossRef] [Green Version]
- Eddingsaas, N.; Pagano, T.; Cummings, C.; Rahman, I.; Robinson, R.; Hensel, E.C. Qualitative Analysis of E-Liquid Emissions as a Function of Flavor Additives Using Two Aerosol Capture Methods. Int. J. Environ. Res. Public Health 2018, 15, 323. [Google Scholar] [CrossRef] [Green Version]
- Hua, M.; Omaiye, E.E.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci. Rep. 2019, 9, 2782. [Google Scholar] [CrossRef] [Green Version]
- Behar, R.Z.; Wang, Y.; Talbot, P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob. Control 2017, 27, 325–333. [Google Scholar] [CrossRef]
- ChemIDplus. A Toxnet Database. Substance Name: Vanillin [NF]. Available online: https://chem.nlm.nih.gov/chemidplus/rn/121-33-5 (accessed on 6 June 2020).
- Clapp, P.W.; Pawlak, E.A.; Lackey, J.T.; Keating, J.E.; Reeber, S.L.; Glish, G.L.; Jaspers, I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Cell. Mol. Physiol. 2017, 313, L278–L292. [Google Scholar] [CrossRef]
- Mathias, C.; Guernsey, L.A.; Zammit, D.; Brammer, C.; Wu, C.A.; Thrall, R.S.; Aguila, H.L. Pro-inflammatory role of natural killer cells in the development of allergic airway disease. Clin. Exp. Allergy 2014, 44, 589–601. [Google Scholar] [CrossRef]
- Culley, F.J. Natural killer cells in infection and inflammation of the lung. Immunology 2009, 128, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, Y.; Kobayashi, K.; Yamamoto, M.; Nakata, K.; Takagawa, T.; Funada, Y.; Kotani, Y.; Karasuyama, H.; Yoshida, M.; Nishimura, Y. Antigen-Specific IgG ameliorates allergic airway inflammation via Fcγ receptor IIB on dendritic cells. Respir. Res. 2011, 12, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, H.Y. Immunoglobulin G and Its Function in the Human Respiratory Tract*. Mayo Clin. Proc. 1988, 63, 161–174. [Google Scholar] [CrossRef]
- De Fonseca, F.R.; Del Arco, I.; Bermúdez-Silva, F.-J.; Bilbao, A.; Cippitelli, A.; Navarro, M. The endocannabinoid system: Physiology and pharmacology. Alcohol Alcohol. 2004, 40, 2–14. [Google Scholar] [CrossRef]
- Cabral, G.A.; Ferreira, G.A.; Jamerson, M.J. Endocannabinoids and the Immune System in Health and Disease. Handb. Exp. Pharmacol. 2015, 231, 185–211. [Google Scholar] [CrossRef]
- Lourbopoulos, A.; Grigoriadis, N.; Lagoudaki, R.; Touloumi, O.; Polyzoidou, E.; Mavromatis, I.; Tascos, N.; Breuer, A.; Ovadia, H.; Karussis, D.; et al. Administration of 2-arachidonoylglycerol ameliorates both acute and chronic experimental autoimmune encephalomyelitis. Brain Res. 2011, 1390, 126–141. [Google Scholar] [CrossRef]
- Krishnan, G.; Chatterjee, N. Endocannabinoids alleviate proinflammatory conditions by modulating innate immune response in muller glia during inflammation. Glia 2012, 60, 1629–1645. [Google Scholar] [CrossRef]
- Lu, Y.; Peng, F.; Dong, M.; Yang, H. Endocannabinoid 2-arachidonylglycerol protects primary cultured neurons against LPS-induced impairments in rat caudate nucleus. J. Mol. Neurosci. 2014, 54, 49–58. [Google Scholar] [CrossRef]
- Panikashvili, D.; Shein, N.A.; Mechoulam, R.; Trembovler, V.; Kohen, R.; Alexandrovich, A.; Shohami, E. The endocannabinoid 2-AG protects the blood–brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines. Neurobiol. Dis. 2006, 22, 257–264. [Google Scholar] [CrossRef]
- Raman, P.; Kaplan, B.L.F.; Kaminski, N.E. 15-Deoxy-Delta(1)(2),(1)(4)-prostaglandin J(2)-glycerol, a putative metabolite of 2-arachidonyl glycerol and a peroxisome proliferator-activated receptor gamma ligand, modulates nuclear factor of activated T cells. J. Pharmacol. Exp. Ther. 2012, 342, 816–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwell, C.E.; Raman, P.; Kaplan, B.L.; Kaminski, N.E. A COX-2 metabolite of the endogenous cannabinoid, 2-arachidonyl glycerol, mediates suppression of IL-2 secretion in activated Jurkat T cells. Biochem. Pharmacol. 2008, 76, 353–361. [Google Scholar] [CrossRef]
- Rockwell, C.E.; Snider, N.T.; Thompson, J.T.; Heuvel, J.P.V.; Kaminski, N.E. Interleukin-2 suppression by 2-arachidonyl glycerol is mediated through peroxisome proliferator-activated receptor gamma independently of cannabinoid receptors 1 and 2. Mol. Pharmacol. 2006, 70, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Nazarewicz, R.R.; Zenebe, W.J.; Parihar, A.; Parihar, M.S.; Vaccaro, M.; Rink, C.; Sen, C.K.; Ghafourifar, P. 12(S)-hydroperoxyeicosatetraenoic acid (12-HETE) increases mitochondrial nitric oxide by increasing intramitochondrial calcium. Arch. Biochem. Biophys. 2007, 468, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Leishangthem, G.D.; Singh, S.; Dinda, A.K.; Biswal, S.; Agrawal, A.; Ghosh, B. 12/15-lipoxygenase expressed in non-epithelial cells causes airway epithelial injury in asthma. Sci. Rep. 2013, 3, srep01540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2012, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, Y.-W.; Han, J.; Liang, H.; Wang, N.; Cheng, Y. 12/15-Lipoxygenase metabolites of arachidonic acid activate PPARγ: A possible neuroprotective effect in ischemic brain. J. Lipid Res. 2015, 56, 502–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scher, J.U.; Pillinger, M.H. The anti-inflammatory effects of prostaglandins. J. Investig. Med. 2009, 57, 703–708. [Google Scholar] [CrossRef]
- Yakar, I.; Melamed, R.; Shakhar, G.; Shakhar, K.; Rosenne, E.; Abudarham, N.; Page, G.G.; Ben-Eliyahu, S. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann. Surg. Oncol. 2003, 10, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.C.; Zhou, X.; Cuchens, M.; Jones, Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J. Immunol. 2001, 166, 885–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grana, R.A.; Popova, L.; Ling, P.M. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Intern. Med. 2014, 174, 812–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutten, L.J.F.; Blake, K.D.; Agunwamba, A.A.; Grana, R.A.; Wilson, P.M.; Ebbert, J.O.; Okamoto, J.M.; Leischow, S.J. Use of E-Cigarettes Among Current Smokers: Associations Among Reasons for Use, Quit Intentions, and Current Tobacco Use. Nicotine Tob. Res. 2015, 17, 1228–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulvers, K.; Hayes, R.B.; Scheuermann, T.S.; Romero, D.R.; Emami, A.S.; Resnicow, K.; Olendzki, E.; Person, S.D.; Ahluwalia, J.S. Tobacco Use, Quitting Behavior, and Health Characteristics Among Current Electronic Cigarette Users in a National Tri-Ethnic Adult Stable Smoker Sample. Nicotine Tob. Res. 2015, 17, 1085–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, A.; Verret, C.M.; Hasan, F.; Lomnicki, S.; Morse, J.; Robichaud, A.; Penn, A. Generation of Electronic Cigarette Aerosol by a Third-Generation Machine-Vaping Device: Application to Toxicological Studies. J. Vis. Exp. 2018. [Google Scholar] [CrossRef] [Green Version]
- Noel, A.; Xiao, R.; Perveen, Z.; Zaman, H.; Le Donne, V.; Penn, A. Sex-specific lung functional changes in adult mice exposed only to second-hand smoke in utero. Respir. Res. 2017, 18, 104. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.R.; Hotten, D.F.; Malakhau, Y.; Volker, E.; Ghio, A.J.; Noble, P.W.; Kraft, M.; Hollingsworth, J.W.; Gunn, M.D.; Tighe, R.M. Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues. Am. J. Respir. Cell Mol. Biol. 2016, 54, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.; Perlman, H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Borazjani, A.; Matthews, A.T.; Mangum, L.C.; Edelmann, M.J.; Ross, M.K. Identification of palmitoyl protein thioesterase 1 in human THP1 monocytes and macrophages and characterization of unique biochemical activities for this enzyme. Biochemistry 2013, 52, 7559–7574. [Google Scholar] [CrossRef] [Green Version]
- Noel, A.; Hansen, S.; Zaman, A.; Perveen, Z.; Pinkston, R.; Hossain, E.; Xiao, R.; Penn, A. In Utero Exposures to Electronic-Cigarette Aerosols Impair the Wnt Signaling during Mouse Lung Development. Am. J. Physiol. Lung Cell Mol. Physiol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Szafran, B.N.; Lee, J.H.; Borazjani, A.; Morrison, P.; Zimmerman, G.; Andrzejewski, K.L.; Ross, M.K.; Kaplan, B.L.F. Characterization of Endocannabinoid-Metabolizing Enzymes in Human Peripheral Blood Mononuclear Cells under Inflammatory Conditions. Molecules 2018, 23, 3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noël, A.; Xiao, R.; Perveen, Z.; Zaman, H.; Rouse, R.; Paulsen, D.; Penn, A. Incomplete lung recovery following sub-acute inhalation of combustion-derived ultrafine particles in mice. Part Fibre Toxicol. 2015, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, A.; Green, J.; Pollard, J.; Tugendreich, S., Jr. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]







| HEPA-Filtered Air | 70% VG/30% PG | 70% VG/30% PG + Vanilla | |
|---|---|---|---|
| Temperature (°C) (± SD) † | 25.5 ± 1.2 | 26.4 ± 4.3 | 24.6 ± 1.6 |
| Relative humidity (%RH) (± SD) † | 69.7 ± 9.3 | 68.0 ± 3.1 | 73.1 ± 4.2 |
| Total particulate matter (TPM) concentration (mg/puff) (± SD) | --- | 0.041 ± 0.031 | 0.035 ± 0.028 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafran, B.N.; Pinkston, R.; Perveen, Z.; Ross, M.K.; Morgan, T.; Paulsen, D.B.; Penn, A.L.; Kaplan, B.L.F.; Noël, A. Electronic-Cigarette Vehicles and Flavoring Affect Lung Function and Immune Responses in a Murine Model. Int. J. Mol. Sci. 2020, 21, 6022. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176022
Szafran BN, Pinkston R, Perveen Z, Ross MK, Morgan T, Paulsen DB, Penn AL, Kaplan BLF, Noël A. Electronic-Cigarette Vehicles and Flavoring Affect Lung Function and Immune Responses in a Murine Model. International Journal of Molecular Sciences. 2020; 21(17):6022. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176022
Chicago/Turabian StyleSzafran, Brittany N., Rakeysha Pinkston, Zakia Perveen, Matthew K. Ross, Timothy Morgan, Daniel B. Paulsen, Arthur L. Penn, Barbara L. F. Kaplan, and Alexandra Noël. 2020. "Electronic-Cigarette Vehicles and Flavoring Affect Lung Function and Immune Responses in a Murine Model" International Journal of Molecular Sciences 21, no. 17: 6022. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176022