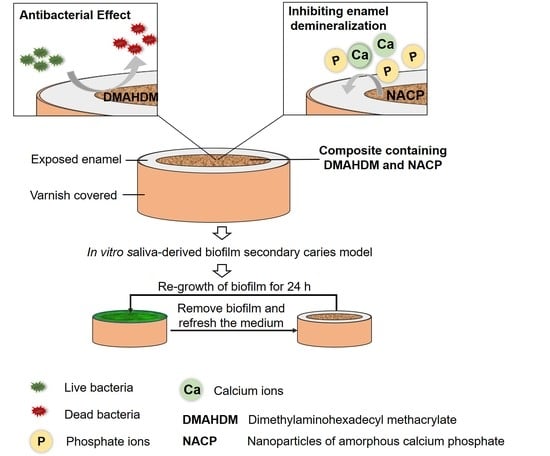
Novel Nanocomposite Inhibiting Caries at the Enamel Restoration Margins in an In Vitro Saliva-Derived Biofilm Secondary Caries Model
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Fabrication of Composites
- (1)
- Heliomolar nanocomposite (referred to as Commercial control);
- (2)
- Experimental composite control. 35% BT + 65% glass particles (referred to as 0DMAHDM + 0NACP control);
- (3)
- Antibacterial composite. 32% BT + 65% glass particles + 3% DMAHDM + 30% NACP (referred to as 3DMAHDM + 0NACP);
- (4)
- Antibacterial and remineralizing composite. 32% BT + 35% glass particles + 3% DMAHDM + 30% NACP (referred to as 3DMAHDM + 30NACP).
4.2. Mechanical Testing
4.3. Composite Disk Preparation for Biofilm Tests
4.4. Bacterial Culture and Biofilm Formation on Composites
4.5. Live/Dead Bacterial Assay
4.6. Biofilms CFU Counts
4.7. MTT Metabolic Assay of Biofilms
4.8. Lactic acid Production by Biofilms
4.9. Water-Insoluble Polysaccharide Production by Biofilms
4.10. Ca and P Ion Release
4.11. Saliva-Derived Biofilm Model for Enamel Demineralization at the Margins
4.12. Hardness Measurement of Enamel at the Margins
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soncini, J.A.; Maserejian, N.N.; Trachtenberg, F.; Tavares, M.; Hayes, C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth: Findings From the New England Children’s Amalgam Trial. J. Am. Dent. Assoc. 2007, 138, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astvaldsdottir, A.; Dagerhamn, J.; van Dijken, J.W.; Naimi-Akbar, A.; Sandborgh-Englund, G.; Tranaeus, S.; Nilsson, M. Longevity of posterior resin composite restorations in adults—A systematic review. J. Dent. 2015, 43, 934–954. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.S.; Alania, Y.; Natale, L.C.; Rodrigues, M.C.; Watts, D.C.; Braga, R.R. Trends in restorative composites research: What is in the future? Braz. Oral Res. 2017, 31, e55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mjor, I.A.; Toffenetti, F. Secondary caries: A literature review with case reports. Quintessence Int. 2000, 31, 165–179. [Google Scholar] [PubMed]
- Irie, M.; Suzuki, K.; Watts, D.C. Marginal gap formation of light-activated restorative materials: Effects of immediate setting shrinkage and bond strength. Dent. Mater. 2002, 18, 203–210. [Google Scholar] [CrossRef]
- Sun, J.; Eidelman, N.; Lin-Gibson, S. 3D mapping of polymerization shrinkage using X-ray micro-computed tomography to predict microleakage. Dent. Mater. 2009, 25, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y.; Tian, F.C.; Niu, L.N.; Ochala, K.; Chen, C.; Fu, B.P.; Wang, X.Y.; Pashley, D.H.; Tay, F.R. Defying ageing: An expectation for dentine bonding with universal adhesives? J. Dent. 2016, 45, 43–52. [Google Scholar] [CrossRef]
- Cenci, M.S.; Pereira-Cenci, T.; Cury, J.A.; Cate, J.M.T. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009, 43, 97–102. [Google Scholar] [CrossRef]
- Yang, Y.; Reipa, V.; Liu, G.; Meng, Y.; Wang, X.; Mineart, K.P.; Prabhu, V.M.; Shi, W.; Lin, N.J.; He, X.; et al. pH-Sensitive Compounds for Selective Inhibition of Acid-Producing Bacteria. ACS Appl. Mater. Interfaces 2018, 10, 8566–8573. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef]
- Imazato, S. Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beyth, N.; Yudovin-Farber, I.; Perez-Davidi, M.; Domb, A.J.; Weiss, E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 22038–22043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, Y.P.; Meghil, M.M.; Pucci, C.R.; Breschi, L.; Pashley, D.H.; Cutler, C.W.; Niu, L.N.; Li, J.Y.; Tay, F.R. Optimizing resin-dentin bond stability using a bioactive adhesive with concomitant antibacterial properties and anti-proteolytic activities. Acta Biomater. 2018, 75, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Söderling, E.; Liu, F.; He, J.; Lassila, L.V.; Vallittu, P.K. Optimizing the concentration of quaternary ammonium dimethacrylate monomer in bis-GMA/TEGDMA dental resin system for antibacterial activity and mechanical properties. J. Mater. Sci. Mater. Med. 2014, 25, 1387–1393. [Google Scholar] [CrossRef]
- Li, F.; Chai, Z.G.; Sun, M.N.; Wang, F.; Ma, S.; Zhang, L.; Fang, M.; Chen, J.H. Anti-biofilm effect of dental adhesive with cationic monomer. J. Dent. Res. 2009, 88, 372–376. [Google Scholar] [CrossRef]
- Beyth, N.; Yudovin-Fearber, I.; Domb, A.J.; Weiss, E.I. Long-term antibacterial surface properties of composite resin incorporating polyethyleneimine nanoparticles. Quintessence Int. 2010, 41, 827–835. [Google Scholar]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Vidal, M.L.; Rego, G.F.; Viana, G.M.; Cabral, L.M.; Souza, J.P.B.; Silikas, N.; Schneider, L.F.; Cavalcante, L.M. Physical and chemical properties of model composites containing quaternary ammonium methacrylates. Dent. Mater. 2018, 34, 143–151. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Weir, M.D.; Zhang, K.; Zhou, Y.; Xu, H.H.K.; Reynolds, M.A. Novel multifunctional dental bonding agent for Class-V restorations to inhibit periodontal biofilms. RSC Adv. 2017, 7, 29004–29014. [Google Scholar] [CrossRef] [Green Version]
- Weir, M.D.; Chow, L.C.; Xu, H.H. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J. Dent. Res. 2012, 91, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrtic, D.; Hailer, A.W.; Takagi, S.; Antonucci, J.M.; Eanes, E.D. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J. Dent. Res. 1996, 75, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, N.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Novel multifunctional dental cement to prevent enamel demineralization near orthodontic brackets. J. Dent. 2017, 64, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, M.D.; Ruan, J.; Zhang, N.; Chow, L.C.; Zhang, K.; Chang, X.; Bai, Y.; Xu, H.H.K. Effect of calcium phosphate nanocomposite on in vitro remineralization of human dentin lesions. Dent. Mater. 2017, 33, 1033–1044. [Google Scholar] [CrossRef]
- Liang, K.; Weir, M.D.; Xie, X.; Wang, L.; Reynolds, M.A.; Li, J.; Xu, H.H. Dentin remineralization in acid challenge environment via PAMAM and calcium phosphate composite. Dent. Mater. 2016, 32, 1429–1440. [Google Scholar] [CrossRef]
- Liang, K.; Zhou, H.; Weir, M.D.; Bao, C.; Reynolds, M.A.; Zhou, X.; Li, J.; Xu, H.H.K. Poly(amido amine) and calcium phosphate nanocomposite remineralization of dentin in acidic solution without calcium phosphate ions. Dent. Mater. 2017, 33, 818–829. [Google Scholar] [CrossRef]
- Melo, M.A.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent. Mater. 2013, 29, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Carrera, C.; Chen, R.; Li, J.; Lenton, P.; Rudney, J.D.; Jones, R.S.; Aparicio, C.; Fok, A. Degradation in the dentin-composite interface subjected to multi-species biofilm challenges. Acta Biomater. 2014, 10, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Rudney, J.D.; Chen, R.; Lenton, P.; Li, J.; Li, Y.; Jones, R.S.; Reilly, C.; Fok, A.S.; Aparicio, C. A reproducible oral microcosm biofilm model for testing dental materials. J. Appl. Microbiol. 2012, 113, 1540–1553. [Google Scholar] [CrossRef] [PubMed]
- Kuper, N.K.; van de Sande, F.H.; Opdam, N.J.; Bronkhorst, E.M.; de Soet, J.J.; Cenci, M.S.; Huysmans, M.C. Restoration materials and secondary caries using an in vitro biofilm model. J. Dent. Res. 2015, 94, 62–68. [Google Scholar] [CrossRef]
- Maia, A.C.; Mangabeira, A.; Vieira, R.; Neves, A.A.; Lopes, R.T.; Pires, T.M.; Viana, G.M.; Cabral, L.M.; Cavalcante, L.M.; Portela, M.B. Experimental composites containing quaternary ammonium methacrylates reduce demineralization at enamel-restoration margins after cariogenic challenge. Dent. Mater. 2019, 35, e175–e183. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.Y.; Li, M.Y. Secondary caries. In Contemporary Approach to Dental Caries; IntechOpen: London, UK, 2012. [Google Scholar]
- Ando, M.; Gonzalez-Cabezas, C.; Isaacs, R.L.; Eckert, G.J.; Stookey, G.K. Evaluation of several techniques for the detection of secondary caries adjacent to amalgam restorations. Caries Res. 2004, 38, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Wright, J.P. Reminova and EAER: Keeping Enamel Whole through Caries Remineralization. Adv. Dent. Res. 2018, 29, 48–54. [Google Scholar] [CrossRef]
- Alsayed, E.Z.; Hariri, I.; Nakashima, S.; Shimada, Y.; Bakhsh, T.A.; Tagami, J.; Sadr, A. Effects of coating materials on nanoindentation hardness of enamel and adjacent areas. Dent. Mater. 2016, 32, 807–816. [Google Scholar] [CrossRef]
- Jokstad, A. Secondary caries and microleakage. Dent. Mater. 2016, 32, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Featherstone, J.D. Remineralization, the natural caries repair process—The need for new approaches. Adv. Dent. Res. 2009, 21, 4–7. [Google Scholar] [CrossRef]
- Silverstone, L.M. Structure of carious enamel, including the early lesion. Oral Sci. Rev. 1973, 3, 100–160. [Google Scholar]
- Fugolin, A.; Pfeifer, C. New resins for dental composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef]
- Faust, D.; Dolado, I.; Cuadrado, A.; Oesch, F.; Weiss, C.; Nebreda, A.R.; Dietrich, C. p38alpha MAPK is required for contact inhibition. Oncogene 2005, 24, 7941–7945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Zhang, K.; Melo, M.A.; Weir, M.D.; Xu, D.J.; Bai, Y.; Xu, H.H. Effects of Long-Term Water-Aging on Novel Anti-Biofilm and Protein-Repellent Dental Composite. Int. J. Mol. Sci. 2017, 18, 186. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Xu, H.H. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Koo, H.; Falsetta, M.L.; Klein, M.I. The exopolysaccharide matrix: A virulence determinant of cariogenic biofilm. J. Dent. Res. 2013, 92, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Ccahuana-Vasquez, R.A.; Tabchoury, C.P.; Tenuta, L.M.; Del Bel Cury, A.A.; Vale, G.C.; Cury, J.A. Effect of frequency of sucrose exposure on dental biofilm composition and enamel demineralization in the presence of fluoride. Caries Res. 2007, 41, 9–15. [Google Scholar] [CrossRef]
- Chen, L.; Ren, Z.; Zhou, X.; Zeng, J.; Zou, J.; Li, Y. Inhibition of Streptococcus mutans biofilm formation, extracellular polysaccharide production, and virulence by an oxazole derivative. Appl. Microbiol. Biotechnol. 2016, 100, 857–867. [Google Scholar] [CrossRef]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials-Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhou, C.C.; Weir, M.D.; Zhou, X.D.; Xu, H.H. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, L.; Xing, D.; Zhang, K.; Weir, M.D.; Liu, H.; Bai, Y.; Xu, H.H.K. Novel dental adhesive with triple benefits of calcium phosphate recharge, protein-repellent and antibacterial functions. Dent. Mater. 2017, 33, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Sun, L.; Chow, L.C.; Xu, H.H. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 98, 80–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomiyama, K.; Mukai, Y.; Kumada, H.; Watanabe, K.; Hamada, N.; Teranaka, T. Formation of subsurface dentin lesions using a polymicrobial biofilm model. Am. J. Dent. 2015, 28, 13–17. [Google Scholar]
- Bridi, E.C.; do Amaral, F.L.; Franca, F.M.; Turssi, C.P.; Basting, R.T. Inhibition of demineralization around the enamel-dentin/restoration interface after dentin pretreatment with TiF4 and self-etching adhesive systems. Clin. Oral Investig. 2016, 20, 857–863. [Google Scholar] [CrossRef]
- Santos, D.; Pires, J.G.; Braga, A.S.; Salomao, P.M.A.; Magalhaes, A.C. Comparison between static and semi-dynamic models for microcosm biofilm formation on dentin. J. Appl. Oral Sci. 2019, 27, e20180163. [Google Scholar] [CrossRef] [Green Version]
- Exterkate, R.A.; Crielaard, W.; Ten Cate, J.M. Different response to amine fluoride by Streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res. 2010, 44, 372–379. [Google Scholar] [CrossRef]
- Sissons, C.H. Artificial dental plaque biofilm model systems. Adv. Dent. Res. 1997, 11, 110–126. [Google Scholar] [CrossRef]
- Li, B.; Zhou, X.; Zhou, X.; Wu, P.; Li, M.; Feng, M.; Peng, X.; Ren, B.; Cheng, L. Effects of different substrates/growth media on microbial community of saliva-derived biofilm. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Huang, X.; Exterkate, R.A.; ten Cate, J.M. Factors associated with alkali production from arginine in dental biofilms. J. Dent. Res. 2012, 91, 1130–1134. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, F.; Azevedo, C.L.; Ferracane, J.L.; Braga, R.R. BisGMA/TEGDMA ratio and filler content effects on shrinkage stress. Dent. Mater. 2011, 27, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.S.; Ferracane, J.L.; Sakaguchi, R.L.; Braga, R.R. Photoinitiator content in restorative composites: Influence on degree of conversion, reaction kinetics, volumetric shrinkage and polymerization stress. Am. J. Dent. 2009, 22, 206–210. [Google Scholar]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xie, X.; Imazato, S.; Weir, M.D.; Reynolds, M.A.; Xu, H.H.K. A protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 702–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Wang, P.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Evaluation of antibacterial and remineralizing nanocomposite and adhesive in rat tooth cavity model. Acta Biomater. 2014, 10, 2804–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khvostenko, D.; Mitchell, J.C.; Hilton, T.J.; Ferracane, J.L.; Kruzic, J.J. Mechanical performance of novel bioactive glass containing dental restorative composites. Dent. Mater. 2013, 29, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Stansbury, J.W.; Bowman, C.N. Impact of curing protocol on conversion and shrinkage stress. J. Dent. Res. 2005, 84, 822–826. [Google Scholar] [CrossRef]
- Xu, X.; Ling, L.; Wang, R.; Burgess, J.O. Formulation and characterization of a novel fluoride-releasing dental composite. Dent. Mater. 2006, 22, 1014–1023. [Google Scholar] [CrossRef]
- Imazato, S.; Ehara, A.; Torii, M.; Ebisu, S. Antibacterial activity of dentine primer containing MDPB after curing. J. Dent. 1998, 26, 267–271. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Melo, M.A.; Weir, M.D.; Zhou, X.; Xu, H.H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Yaskell, T.; Klepac-Ceraj, V.; Lynch, M.C.; Soukos, N.S. Antimicrobial action of minocycline microspheres versus 810-nm diode laser on human dental plaque microcosm biofilms. J. Periodontol. 2014, 85, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Weir, M.D.; Chow, L.C.; Antonucci, J.M.; Chen, J.; Xu, H.H. Novel rechargeable calcium phosphate dental nanocomposite. Dent. Mater. 2016, 32, 285–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Peng, X.; Zhou, X.; Weir, M.D.; Melo, M.A.S.; Tay, F.R.; Imazato, S.; Oates, T.W.; Cheng, L.; Xu, H.H.K. In vitro evaluation of composite containing DMAHDM and calcium phosphate nanoparticles on recurrent caries inhibition at bovine enamel-restoration margins. Dent. Mater. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Buetas, E.; Rosier, B.; Mazurel, D.; Villanueva-Castellote, A.; Llena, C.; Ferrer, M.D. Development of an in vitro system to study oral biofilms in real time through impedance technology: Validation and potential applications. J. Oral Microbiol. 2019, 11, 1609838. [Google Scholar] [CrossRef]
- Medeiros, M.I.D.; Carlo, H.L.; Santos, R.L.D.; Sousa, F.B.; Castro, R.D.; Franca, R.C.S.; Carvalho, F.G. TiF4 varnish protects the retention of brackets to enamel after in vitro mild erosive challenge. J. Appl. Oral Sci. 2018, 26, e20170222. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Peng, X.; Zhou, X.; Bonavente, A.; Weir, M.D.; Melo, M.A.S.; Imazato, S.; Oates, T.W.; Cheng, L.; Xu, H.H.K. Novel Nanocomposite Inhibiting Caries at the Enamel Restoration Margins in an In Vitro Saliva-Derived Biofilm Secondary Caries Model. Int. J. Mol. Sci. 2020, 21, 6369. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176369
Zhou W, Peng X, Zhou X, Bonavente A, Weir MD, Melo MAS, Imazato S, Oates TW, Cheng L, Xu HHK. Novel Nanocomposite Inhibiting Caries at the Enamel Restoration Margins in an In Vitro Saliva-Derived Biofilm Secondary Caries Model. International Journal of Molecular Sciences. 2020; 21(17):6369. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176369
Chicago/Turabian StyleZhou, Wen, Xinyu Peng, Xuedong Zhou, Andrea Bonavente, Michael D. Weir, Mary Anne S. Melo, Satoshi Imazato, Thomas W. Oates, Lei Cheng, and Hockin H. K. Xu. 2020. "Novel Nanocomposite Inhibiting Caries at the Enamel Restoration Margins in an In Vitro Saliva-Derived Biofilm Secondary Caries Model" International Journal of Molecular Sciences 21, no. 17: 6369. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21176369