Functional Grading of a Transversely Isotropic Hyperelastic Model with Applications in Modeling Tricuspid and Mitral Valve Transition Regions
Abstract
:1. Introduction
1.1. Background and Motivation
1.2. Prior Work in Constitutive Modeling of Anisotropic Soft-Tissue
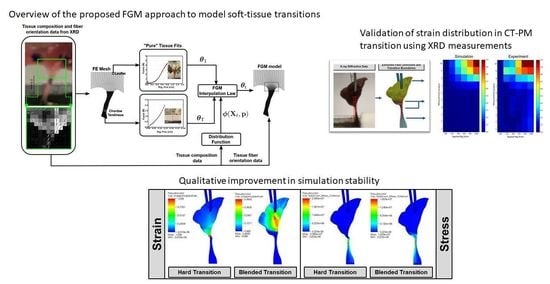
1.3. Problem Statement and Scope
- 1.
- FGM interpolation problem for the TI model: Given the TI model fits represented by and for the terminal materials at either side of the transition which are assumed to be homogeneous (“pure” tissue), and a user-defined distribution function represented by shape parameters , formulate an interpolation law to obtain functionally graded TI parameters at transition layer t with coordinates given by , where .
- 2.
- Shape parameter estimation problem: Given experimental data on the constituent composition and molecular strain at the transition region, obtain the optimal shape parameter of the distribution function using optimization techniques.
2. Materials and Methods
2.1. FGM Modeling
2.1.1. TI Model Constitutive Law
- 1.
- , : These correspond to the isotropic stiffness of the matrix component of the material.
- 2.
- , , : These correspond to the fiber component of the material and are used to model the exponential toe region of the stress–strain curve. and control the degree of exponential rise, while the critical fiber stretch, , controls the extent of the exponential regime (see Figure 2a).
- 3.
- , : These correspond to the post-exponential linear stretching of the fiber. represents the modulus of straightened fibers.
2.1.2. Functional Grading of the TI Model
2.1.3. Interpolation Law for Functional Grading of TI Model
| Algorithm 1 FGM Interpolation Law for the TI model |
|
- 1.
- Case 1. All TI parameters except are interpolated individually with represented by a symmetric sigmoid (The explicit expression for the sigmoid is given later in Section 2.4.2—see Equation (21)). is computed by enforcing continuity at and using the other interpolated parameters.
- 2.
- Case 2. All TI parameters are interpolated using used in Case 1.
- 3.
- Case 3: TI parameters interpolated with Algorithm 1 using , as in Cases 1 and 2. Without loss of generality, the function for interpolating the parameter and the discontinuity index f is set to , since a sigmoid is by definition monotonic, i.e., . The resulting TI parameter distribution is shown in Figure 3a.
2.2. Experimental Methods
2.3. Virtual Matched Pair FE Mesh Setup
2.4. FE Simulation Setup and Parameter Estimation
2.4.1. “Pure” CT, LL and PM Characterization
2.4.2. CT–LL transition
- 1.
- First, the experimental specimen frame was rigidly registered to the FE mesh of the CT–LL specimen.
- 2.
- After registration, the collagen orientations in the transition region obtained from XRD were mapped to the corresponding mesh by assigning to each element of the mesh the corresponding collagen orientation using a KD-tree based nearest-neighbor search.
- 3.
- Fiber orientations were subsequently propagated to the whole CT–LL mesh (regions outside of the scanned region of the specimen), by extrapolating the XRD orientation data.
2.4.3. CT–PM Transition
2.5. Numerical Implementation
3. Results
3.1. FGM Model Validation in CT–LL Transition
3.2. FGM Model Validation in CT–PM Transition
4. Discussion
5. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TI | Transverse isotropy/transversely isotropic |
| FGM | Functionally graded material |
| FE | Finite element |
| MV | Mitral valve |
| TV | Tricuspid valve |
| LL | Leaflet |
| PM | Papillary muscle |
| CT | Chordae tendinae |
| XRD | X-Ray diffraction |
| MTJ | Muscle–tendon junction |
| CAD | Computer-aided design |
References
- Olasky, J.; Sankaranarayanan, G.; Seymour, N.; Magee, J.; Enquobahrie, A.; Lin, M.; Aggarwal, R.; Brunt, L.; Schwaitzberg, S.; Cao, C.G.; et al. Identifying opportunities for virtual reality simulation in surgical education: A review of the proceedings from the innovation, design, and emerging alliances in surgery (IDEAS) conference: VR surgery. Surg. Innov. 2015, 22, 514–521. [Google Scholar] [CrossRef]
- Park, G.; Kim, T.; Panzer, M.; Crandall, J. Validation of shoulder response of human body finite-element model (GHBMC) under whole body lateral impact condition. Ann. Biomed. Eng. 2016, 44, 2558–2576. [Google Scholar] [CrossRef] [PubMed]
- Butz, K.; Spurlock, C.; Roy, R.; Bell, W.; Barrett, P.; Ward, A.; Xiao, X.; Shirley, A.; Welch, C.; Lister, K. Development of the CAVEMAN human body model: Validation of lower extremity sub-injurious response to vertical accelerative loading. Stapp Car Crash J. 2017, 61, 175–209. [Google Scholar] [PubMed]
- Tidball, J. 12 Myotendinous Junction Injury in Relation to Junction Structure and Molecular Composition. Exerc. Sport Sci. Rev. 1991, 19, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Sedransk, K.; Grande-Allen, K.; Vesely, I. Failure mechanics of mitral valve chordae tendineae. J. Heart Valve Dis. 2002, 11, 644–650. [Google Scholar]
- Hedia, H.; Mahmoud, N. Design optimization of functionally graded dental implant. Bio-Med. Mater. Eng. 2004, 14, 133–143. [Google Scholar]
- Leong, K.; Chua, S.; Sudarmadji, N.; Yeong, W. Engineering functionally graded tissue engineering scaffolds. J. Mech. Behav. Biomed. 2008, 1, 140–152. [Google Scholar] [CrossRef]
- Charvet, B.; Ruggiero, F.; Guellec, D.L. The development of the myotendinous junction: A review. Muscles Ligaments Tendons J. 2012, 2, 53. [Google Scholar]
- Liao, J.; Vesely, I. Relationship between collagen fibrils, glycosaminoglycans, and stress relaxation in mitral valve chordae tendineae. Ann. Biomed. Eng. 2004, 32, 977–983. [Google Scholar] [CrossRef]
- Herring, S.; Grimm, A.; Grimm, B. Regulation of sarcomere number in skeletal muscle: A comparison of hypotheses. Muscle Nerve 1984, 7, 161–173. [Google Scholar] [CrossRef]
- Avazmohammadi, R.; Soares, J.; Li, D.; Raut, S.; Gorman, R.; Sacks, M. A contemporary look at biomechanical models of myocardium. Annu. Rev. Biomed. Eng. 2019, 21, 417–442. [Google Scholar] [CrossRef] [PubMed]
- Prot, V.; Skallerud, B.; Holzapfel, G. Transversely isotropic membrane shells with application to mitral valve mechanics. Constitutive modelling and finite element implementation. Int. J. Numer. Meth. Eng. 2007, 71, 987–1008. [Google Scholar] [CrossRef]
- Prot, V.; Skallerud, B.; Holzapfel, G. On modelling and analysis of healthy and pathological human mitral valves: Two case studies. J. Mech. Behav. Biomed. 2010, 3, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Strumpf, R.; Chew, P.; Zeger, S. Quantification of the mechanical properties of noncontracting canine myocardium under simultaneous biaxial loading. J. Biomech. 1987, 20, 577–589. [Google Scholar] [CrossRef]
- Sun, W.; Abad, A.; Sacks, M. Simulated bioprosthetic heart valve deformation under quasi-static loading. J. Biomech. Eng. 2005, 127, 905. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.; Holmes, J.; McCulloch, A. Modelling cardiac mechanical properties in three dimensions. Philos. Trans. R. Soc. A 2001, 359, 1233–1250. [Google Scholar] [CrossRef]
- Sun, W.; Sacks, M. Finite element implementation of a generalized Fung-elastic constitutive model for planar soft tissues. Biomech. Model. Mechanobiol. 2005, 4, 190–199. [Google Scholar] [CrossRef]
- Pope, A.; Sands, G.; Smaill, B.; LeGrice, I. Three-dimensional transmural organization of perimysial collagen in the heart. Am. J. Physiol.-Heart C 2008, 295, H1243–H1252. [Google Scholar] [CrossRef]
- Billiar, K.; Kristen, L.; Sacks, M. Biaxial mechanical properties of the native and glutaraldehyde-treated aortic valve cusp: Part II—A structural constitutive model. J. Biomech. Eng. 2000, 122, 327–335. [Google Scholar] [CrossRef]
- Freed, A.; Einstein, D.; Vesely, I. Invariant formulation for dispersed transverse isotropy in aortic heart valves. Biomech. Model. Mechanobiol. 2005, 4, 100–117. [Google Scholar] [CrossRef]
- Sacks, M. Biaxial mechanical evaluation of planar biological materials. J. Elast. 2000, 61, 199. [Google Scholar] [CrossRef]
- Toma, M.; Jensen, M.; Einstein, D.; Yoganathan, A.; Cochran, R.; Kunzelman, K. Fluid–structure interaction analysis of papillary muscle forces using a comprehensive mitral valve model with 3D chordal structure. Ann. Biomed. Eng. 2016, 44, 942–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunzelman, K.; Einstein, D.; Cochran, R. Fluid–structure interaction models of the mitral valve: Function in normal and pathological states. Philos. Trans. R. Soc. B 2007, 362, 1393–1406. [Google Scholar] [CrossRef] [Green Version]
- Hunter, P.; Nash, M.; Sands, G. Computational Electromechanics of the Heart. In Computational Biology of the Heart; Wiley: West Sussex, England, 1997; pp. 345–407. [Google Scholar]
- Holzapfel, G.; Ogden, R. Constitutive modelling of passive myocardium: A structurally based framework for material characterization. Philos. Trans. R. Soc. A 2009, 367, 3445–3475. [Google Scholar] [CrossRef] [PubMed]
- Gasser, C.; Ogden, R.; Holzapfel, G. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 2006, 3, 15–35. [Google Scholar] [CrossRef]
- Kroon, M.; Holzapfel, G. A new constitutive model for multi-layered collagenous tissues. J. Biomech. 2008, 41, 2766–2771. [Google Scholar] [CrossRef]
- Zhang, W.; Ayoub, S.; Liao, J.; Sacks, M.S. A meso-scale layer-specific structural constitutive model of the mitral heart valve leaflets. Acta Biomater. 2016, 32, 238–255. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Martin, C.; Pham, T. Computational modeling of cardiac valve function and intervention. Annu. Rev. Biomed. Eng. 2014, 16, 53–76. [Google Scholar] [CrossRef] [Green Version]
- Sacks, M.; Sun, W. Multiaxial mechanical behavior of biological materials. Annu. Rev. Biomed. Eng. 2003, 5, 251–284. [Google Scholar] [CrossRef]
- Chen, L.; Yin, F.; May-Newman, K. The structure and mechanical properties of the mitral valve leaflet-strut chordae transition zone. J. Biomech. Eng. 2004, 126, 244–251. [Google Scholar] [CrossRef]
- Rego, B.; Sacks, M. A functionally graded material model for the transmural stress distribution of the aortic valve leaflet. J. Biomech. 2017, 54, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baillargeon, B.; Rebelo, N.; Fox, D.; Taylor, R.; Kuhl, E. The living heart project: A robust and integrative simulator for human heart function. Eur. J. Mech. A Solid 2014, 48, 38–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Feng, L.; Qi, N.; Berry, C.; Griffith, B.; Luo, X. A coupled mitral valve—Left ventricle model with fluid–structure interaction. Med. Eng. Phys. 2017, 47, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Vavalle, N.; Moreno, D.; Stitzel, J.; Gayzik, S. Validation of simulated chestband data in frontal and lateral loading using a human body finite element model. Traffic Inj. Prev. 2014, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Newell, N.; Salzar, R.; Bull, A.; Masouros, S. A validated numerical model of a lower limb surrogate to investigate injuries caused by under-vehicle explosions. J. Biomech. 2016, 49, 710–717. [Google Scholar] [CrossRef]
- Schwartz, D.; Guleyupoglu, B.; Koya, B.; Stitzel, J.; Gayzik, S. Development of a computationally efficient full human body finite element model. Traffic Inj. Prev. 2015, 16, S49–S56. [Google Scholar] [CrossRef]
- Weiss, J.; Maker, B.; Govindjee, S. Finite element implementation of incompressible, transversely isotropic hyperelasticity. Comp. Method Appl. M. 1996, 135, 107–128. [Google Scholar] [CrossRef]
- Pena, E.; Calvo, B.; Martinez, M.; Doblare, M. A three-dimensional finite element analysis of the combined behavior of ligaments and menisci in the healthy human knee joint. J. Biomech. 2006, 39, 1686–1701. [Google Scholar] [CrossRef]
- Weiss, J. A Constitutive Model and Finite Element Representation for Transversely Isotropic Soft Tissues. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 1995. [Google Scholar]
- Shim, V.B.; Fernandez, J.W.; Gamage, P.B.; Regnery, C.; Smith, D.W.; Gardiner, B.S.; Lloyd, D.G.; Besier, T. Subject-specific finite element analysis to characterize the influence of geometry and material properties in Achilles tendon rupture. J. Biomech. 2014, 47, 3598–3604. [Google Scholar] [CrossRef]
- Hallquist, J. LS-DYNA Theory Manual. Available online: http://www.lstc.com/pdf/ls-dyna_theory_manual_2006.pdf (accessed on 4 September 2020).
- Maas, S.; Ellis, B.; Ateshian, G.; Weiss, J. FEBio: Finite elements for biomechanics. J. Biomech. Eng. 2012, 134, 011005. [Google Scholar] [PubMed]
- Madhurapantula, R.; Krell, G.; Morfin, B.; Roy, R.; Lister, K.; Orgel, J. Advanced methodology and preliminary measurements of molecular and mechanical properties of heart valves under dynamic strain. Int. J. Mol. Sci. 2020, 21, 763. [Google Scholar] [CrossRef] [Green Version]
- Chi, S.; Chung, Y. Mechanical behavior of functionally graded material plates under transverse load—Part I: Analysis. Int. J. Solids Struct. 2006, 43, 3657–3674. [Google Scholar] [CrossRef] [Green Version]
- Anani, Y.; Rahimi, G. Stress analysis of thick pressure vessel composed of functionally graded incompressible hyperelastic materials. Int. J. Mech. Sci. 2015, 104, 1–7. [Google Scholar] [CrossRef]
- Madhurapantula, R.; Eidsmore, A.; Modrich, C.; Orgel, J. New Methodology and Preliminary Data in the Characterization of the Muscle Tendon Junction of Mammalian Muscle Tissues. EMS Eng. Sci. J. 2017, 1, 1–7. [Google Scholar]
- Adams, B.; Bohnhoff, W.; Dalbey, K.; Eddy, J.; Eldred, M.; Gay, D.; Haskell, K.; Hough, P.; Swiler, L. DAKOTA, A Multilevel Parallel Object-Oriented Framework for Design Optimization, Parameter Estimation, Uncertainty Quantification, and Sensitivity Analysis: Version 5.0 User’s Manual; Tech. Rep. SAND2010-2183; Sandia National Laboratories: Albuquerque, NM, USA, 2009.
- Chi, S.; Chung, Y. Mechanical behavior of functionally graded material plates under transverse load—Part II: Numerical results. Int. J. Solids Struct 2006, 43, 3675–3691. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, F.; Hofmann, F.; Smith, A.; Thompson, M. Probing multi-scale mechanical damage in connective tissues using X-ray diffraction. Acta Biomater. 2016, 45, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Zhang, W.; Liao, J.; Carruthers, C.; Sacks, J.; Sacks, M. On the presence of affine fibril and fiber kinematics in the mitral valve anterior leaflet. Biophys. J. 2015, 108, 2074–2087. [Google Scholar] [CrossRef] [Green Version]
- Billiar, K.; Sacks, M. A method to quantify the fiber kinematics of planar tissues under biaxial stretch. J. Biomech. 1997, 30, 753–756. [Google Scholar] [CrossRef]
- Liao, J.; Yang, L.; Grashow, J.; Sacks, M. The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J. Biomech. Eng. 2007, 129, 78–87. [Google Scholar]
- Varma, S.; Orgel, J.; Schieber, J. Nanomechanics of type I collagen. Biophys. J. 2016, 111, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratzl, P.; Misof, K.; Zizak, I.; Rapp, G.; Amenitsch, H.; Bernstorff, S. Fibrillar structure and mechanical properties of collagen. J. Struct. Biol. 1998, 122, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Neice, R. Development and Validation of a Pelvis Finite Element Model for Side Panel Intrusion Threats. Master’s Thesis, University of Virginia, Charlottesville, VA, USA, 2019. [Google Scholar]
- Holzapfel, G.; Gasser, T.; Ogden, R. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 2000, 61, 1–48. [Google Scholar] [CrossRef]
- Solid 3D Human Heart Model. Available online: https://www.zygote.com/cad-models/solid-3d-human-anatomy/solid-3d-human-heart (accessed on 3 September 2020).
- Holzapfel, G.; Ogden, R. Constitutive modelling of arteries. Philos. Trans. R. Soc. A 2010, 466, 1551–1597. [Google Scholar] [CrossRef]
- Feng, Y.; Okamoto, R.; Genin, G.; Bayly, P. On the accuracy and fitting of transversely isotropic material models. J. Mech. Behav. Biomed. 2016, 61, 554–566. [Google Scholar] [CrossRef] [Green Version]
- Orgel, J.; Miller, A.; Irving, T.; Fischetti, R.; Hammersley, A.; Wess, T. The in situ supermolecular structure of type I collagen. Structure 2001, 9, 1061–1069. [Google Scholar] [CrossRef] [Green Version]
- Orgel, J.; Irving, T. Advances in Fiber Diffraction of Macromolecular Assembles. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Wiley: Hoboken, NJ, USA, 2014; pp. 1–26. [Google Scholar]
- Genin, G.; Kent, A.; Birman, V.; Wopenka, B.; Pasteris, J.; Marquez, P.; Thomopoulos, S. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys. J. 2009, 97, 976–985. [Google Scholar] [CrossRef] [Green Version]










| Porcine TV | |||||||
| CT | 3.00 × 10 | 0.0 | 0.70 × 10 | 80.00 | 5.441 × 10 | 1.086 | 0.996 |
| LL | 1.95 × 10 | 0.0 | 1.00 × 10 | 13.50 | 4.80 × 10 | 1.28 | 0.995 |
| PM | 0.30 × 10 | 0.0 | 0.50 × 10 | 28.50 | 1.024 × 10 | 1.15 | 0.814 |
| Porcine MV | |||||||
| CT | 3.37 × 10 | 0.0 | 8.82 × 10 | 60.00 | 9.21 × 10 | 1.086 | 0.991 |
| LL | 3.00 × 10 | 0.0 | 9.00 × 10 | 40.00 | 5.38 × 10 | 1.010 | 0.956 |
| PM | 1.05 × 10 | 0.0 | 0.50 × 10 | 24.50 | 1.111 × 10 | 1.09 | 0.996 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, R.; Warren, E., Jr.; Xu, Y.; Yow, C.; Madhurapantula, R.S.; Orgel, J.P.R.O.; Lister, K. Functional Grading of a Transversely Isotropic Hyperelastic Model with Applications in Modeling Tricuspid and Mitral Valve Transition Regions. Int. J. Mol. Sci. 2020, 21, 6503. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186503
Roy R, Warren E Jr., Xu Y, Yow C, Madhurapantula RS, Orgel JPRO, Lister K. Functional Grading of a Transversely Isotropic Hyperelastic Model with Applications in Modeling Tricuspid and Mitral Valve Transition Regions. International Journal of Molecular Sciences. 2020; 21(18):6503. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186503
Chicago/Turabian StyleRoy, Rajarshi, Eric Warren, Jr., Yaoyao Xu, Caleb Yow, Rama S. Madhurapantula, Joseph P. R. O. Orgel, and Kevin Lister. 2020. "Functional Grading of a Transversely Isotropic Hyperelastic Model with Applications in Modeling Tricuspid and Mitral Valve Transition Regions" International Journal of Molecular Sciences 21, no. 18: 6503. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186503