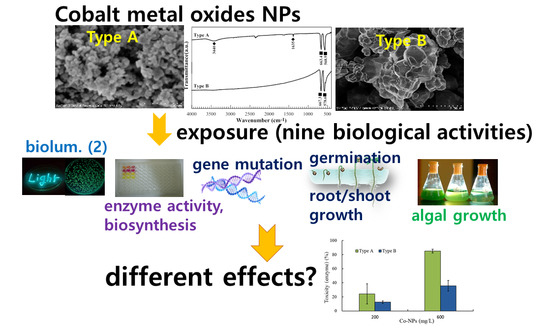
Comparative Effects of Particle Sizes of Cobalt Nanoparticles to Nine Biological Activities
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Tested Co-NPs
2.2. Effects of Co-NPs on the Activities of Various Bacterial Systems
2.3. Effects of Co-NPs on Gene Mutation
2.4. Effects of Co-NPs on Algal Growth
2.5. Effects of Co-NPs on Seed Germination and Root/Shoot Growth
3. Discussion
3.1. Characterization of Tested Co-NPs
3.2. Effects on Five Bacterial Activities
3.3. Effects on Algal Growth
3.4. Effects on Germination and Root/Shoot Growth
3.5. Overall Effects on the Biological Activities
4. Materials and Methods
4.1. Characterization of NPs, Chemicals, and Determination of Cobalt in Solution
4.2. Toxicity Bioassay Assessment
4.3. Effects of Co-NPs on Bacterial Bioluminescence Activity
4.4. Effects of Co-NPs on Enzymatic Activity and Enzyme Biosynthesis
4.5. Effects of Co-NPs on Bacterial Gene Mutation
4.6. Effects of Co-NPs on Algal Growth Activity
4.7. Effects of Co-NPs on Seed Germination and Root/Shoot Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alinovi, R.; Goldoni, M.; Pinelli, S.; Campanini, S.; Aliatis, M.I.; Bersani, D.; Lottici, P.P.; Iavicoli, S.; Petyx, M.; Mozzoni, P.; et al. Oxidative and pro-inflammatory effects of cobalt and titanium oxide nanoparticles on aortic and venous endothelial cells. Toxicol. In Vitro 2015, 29, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Bossi, E.; Zanella, D.; Gornati, R.; Nernardini, G. Cobalt oxide nanoparticles can enter inside the cells by crossing plasma membranes. Sci. Rep. 2016, 6, 22254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Zhang, L.; Zhao, J.; Xing, B. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ. Sci. Nano 2016, 3, 240–255. [Google Scholar] [CrossRef]
- Abudayyak, M.; Gurkaynak, T.A.; Ozhan, G. In vitro evaluation of cobalt oxide nanoparticle-induced toxicity. Toxicol. Indust. Health 2017, 33, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.; Bresson, C.; Carolles, C.; Gautier, C.; Roudeau, S.; Perrin, L.; Janin, M.; Floriani, M.; Aloin, V.; Carmona, A.; et al. Low-solubility particles and a Trojan-horse type mechanism of toxicity: the case of cobalt oxide on human lung cells. Part. Fibre Toxicol. 2014, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Ates, M.; Demir, V.; Arslan, Z.; Camas, M.; Celik, F. Toxicity of engineered nickel oxide and cobalt oxide nanoparticles to Artemia salina in seawater. Wat. Air Soil Pollut. 2016, 227, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, W.; Minami, K.; Shrestha, L.K.; Ji, Q.; Hill, J.P.; Ariga, K. Bioactive nanocarbon assemblies: Nanoarchitectonics and applications. Nano Today 2014, 9, 378–394. [Google Scholar] [CrossRef] [Green Version]
- Magaye, R.; Zhao, J.; Bowman, L.; Ding, M. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles. Exp. Ther. Med. 2012, 4, 551–561. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, K.; Cramer, H.; Albrecht, C.; Schins, R.; Zimmermann, U.; Dopp, E. Vanadium pentoxide-coated ultrafine titanium dioxide particles induce cellular damage and micronucleus formation in V79 cells. J. Toxicol. Environ. Health A 2008, 71, 976–980. [Google Scholar] [CrossRef]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef]
- He, X.; Aker, W.G.; Fu, P.P.; Hwang, H.M. Toxicity of engineered metal oxide nanomaterials mediated by nano-bio-eco-interactions: A review and perspective. Environ. Sci. Nano 2015, 2, 564–582. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Grassian, V.H. Biological and environmental media control oxide nanoparticle surface composition: The roles of biological components (proteins and amino acids), inorganic oxyanions and humic acid. Environ. Sci. Nano 2015, 2, 429–439. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Dash, S.K.; Tripathy, S.; Das, B.; Mandal, D.; Pramanik, P.; Roy, S. Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chem. Biol. Interact. 2015, 226, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Monikh, F.A.; Arenas-Lago, D.; Porcal, P.; Grillo, R.; Zhang, P.; Guo, Z.; Vijver, M.G.; Peijnenburg, W.J.G.M. Do the joint effects of size, shape and ecocorona influence the attachment and physical eco(cyto)toxicity of nanoparticles to algae? Nanotoxicology 2020, 14, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 1–21. [Google Scholar] [CrossRef]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Aruoja, V.; Dubourguier, H.C.; Kasemets, K.; Kahru, A. Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci. Total Environ. 2009, 407, 1461–1468. [Google Scholar] [CrossRef]
- Di Salvatore, M.; Carafa, A.M.; Carrtu, G. Assessment of heavy metals phytotoxicity using seed germination and root elongation tests: A comparison of two growth substrates. Chemosphere 2008, 73, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. 2000, 455, 29–60. [Google Scholar] [CrossRef]
- Landsiedel, R.; Kapp, M.D.; Schulz, M.; Wiench, K.; Oesch, F. Genotoxicity investigations on nanomaterials: Methods, preparation and characterization of test material, potential artifacts and limitations–Many questions, some answers. Mutat. Res. 2009, 681, 241–258. [Google Scholar] [CrossRef]
- Makhlouf, S.A.; Bakr, Z.H.; Aly, K.I.; Moustafa, M.S. Structural, electrical and optical properties of Co3O4 nanoparticles. Superlatti. Microstruct. 2013, 64, 107–117. [Google Scholar] [CrossRef]
- Farhadi, S.; Safabakhsh, J.; Zaringhadam, P. Synthesis, characterization, and investigation of optical and magnetic properties of cobalt oxide (CO3O4) nanoparticles. J. Nanostruct. Chem. 2013, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Farhadi, S.; Javanmard, M.; Nadri, G. Characterization of cobalt oxide nanoparticles prepared by the thermal decomposition of [Co(NH3)5(H2O)](NO3)3 complex and study of their photocatalytic activity. Acta. Chim. Slov. 2016, 63, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles in aqueous media. Environ. Pollut. 2014, 191, 132–138. [Google Scholar] [CrossRef]
- Raman, V.; Suresh, S.; Savarimuthu, P.; Raman, T.; Tsatsakis, A.M.; Golokhvast, K.S.; Vadivel, V.K. Synthesis of Co3O4 nanoparticles with block and sphere morphology, and investigation into the influence of morphology on biological toxicity. Exp. Ther. Med. 2016, 11, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, F.; Yaqub, A.; Tanvir, S.; Anderson, W.A. Poly-L-arginine coated silver nanoprisms and their anti-bacterial properties. Nanomaterials 2017, 7, 296. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y.; Lang, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early developmental stage. J. Environ. Sci. Health A 2008, 43, 278–284. [Google Scholar] [CrossRef]
- Papis, E.; Rossi, F.; Raspanti, M.; Dalle-Donne, I.; Colombo, G.; Milzani, A.; Bernardini, G.; Gornati, R. Engineered cobalt oxide nanoparticles readily enter cells. Toxicol. Lett. 2009, 189, 253–259. [Google Scholar] [CrossRef]
- Al-Bairuty, G.A.; Boyle, D.; Henry, T.B.; Handy, R.D. Sublethal effects of copper sulphate compared to copper nanoparticles in rainbow trout (Oncorhynchus mykiss) at low pH: physiology and metal accumulation. Aquat. Toxicol. 2016, 174, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Razmara, P.; Lari, E.; Mohaddes, E.; Zhang, Y.G.; Goss, G.; Pyle, G.G. The effect of copper nanoparticles on olfaction in rainbow trout (Oncorhynchus mykiss). Environ. Sci. Nano 2019, 6, 2094–2104. [Google Scholar] [CrossRef]
- Li, M.; Nan, L.; Liang, C.; Sun, Z.; Yang, L.; Yang, K. Antibacterial behavior and related mechanisms of martensitic Cu-bearing stainless steel evaluated by a mixed infection model of Escherichia coli and Staphylococcus aureus in vitro. J. Mater. Sci. Technol. 2021, 62, 139–147. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, L.; Zhu, J.; Wu, H.; Li, W.; Sun, Q. Antibacterial properties and mechanism of biopolymer-based films functionalized by CuO/ZnO nanoparticles against Escherichia coli and Staphylococcus aureus. J. Haz. Mater. 2021, 402, 123542. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.S.; Griffiths, S.M.; Williams, O.M.; Maffeis, T.G.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.J.; Quigg, E.A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [Green Version]
- Dash, A.; Singh, A.P.; Chaudhary, B.R.; Singh, S.K.; Dash, D. Effect of silver nanoparticles on growth of eukaryotic green algae. Nano-Micro Lett. 2012, 4, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Hund-Rinke, K.; Simon, M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ. Sci. Pollut. Res. 2006, 13, 225–232. [Google Scholar] [CrossRef]
- Miao, A.J.; Schwehr, K.A.; Xu, C.; Zhang, S.J.; Luo, Z.; Quigg, A.; Santschi, P.H. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 2009, 157, 3034–3041. [Google Scholar] [CrossRef]
- Pikula, K.; Mintcheva, N.; Kulinich, S.A.; Zakharenko, A.; Markina, Z.; Chaika, V.; Orlova, T.; Mezhuev, Y.; Kokkinakis, E.; Tsatsakis, A.; et al. Aquatic toxicity and mode of action of CdS and ZnS nanoparticles in four microalgae species. Environ. Res. 2020, 186, 109513. [Google Scholar] [CrossRef] [PubMed]
- El-Temsah, Y.S.; Joner, E.J. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ. Toxicol. 2012, 27, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Akinci, I.E.; Akinci, S. Effect of chromium toxicity on germination and early seedling growth in melon (Cucumis melo L.). Afr. J. Biotechnol. 2010, 9, 4589–4594. [Google Scholar]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.M.; An, Y.J.; Yoon, H.K.; Kweon, H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiates) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Environ. Toxicol. Chem. 2008, 27, 103–112. [Google Scholar] [CrossRef]
- Srivastava, N. Interaction of cobalt nanoparticles with plants: a cytogenetical aspect. J. Exp. Nanosci. 2015, 10, 769–776. [Google Scholar] [CrossRef] [Green Version]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Crane, M.; Handy, R.D.; Garrod, J.; Owen, R. Ecotoxicity test methods and environmental hazard assessment for engineered nanoparticles. Ecotoxicology 2008, 17, 421–437. [Google Scholar] [CrossRef]
- Menard, A.; Drobne, D.; Jemec, A. Ecotoxicity of nanosized TiO2. Review of in vivo data. Environ. Pollut. 2011, 159, 677–684. [Google Scholar] [CrossRef]
- Liu, Y.; Baas, J.; Peijnenburg, W.G.M.; Vijver, M.G. Evaluating the combined toxicity of Cu and ZnO nanoparticles: Utility of the concept of additivity and a nested experimental design. Environ. Sci. Technol. 2016, 50, 5328–5337. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Tan, W.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Ji, R.; Yin, Y.; Guo, H. Interaction of metal oxide nanoparticles with higher terrestrial plants: Physiological and biochemical aspects. Plant Physiol. Biochem. 2016, 110, 210–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, I.C.; Raliya, R.; Ko, K.S.; Biswas, P. ZnO nanoparticles: Effect of size on bacterial bioluminescence, seed germination, algal growth, and gene mutation. Environ. Eng. Sci. 2018, 35, 231–239. [Google Scholar] [CrossRef]
- Kong, I.C.; Suh, H.; Yang, S.; Burlage, R.S. A bioluminescence reporter strain utilizing the lower pathway promoter (Pm) of the xyl operon of Pseudomonas: optimization of a bioassay for m-toluate. Adv. Environ Res. 2004, 8, 647–654. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, K.J.; Cho, J.W.; Jeon, S.L.; Kang, S.H. A study on comparison and analysis of chlorophyll sensor with aceton extraction for chlorophyll measurement in the Nakdong River. J. Korean Soc. Wat. Wastewat. 2015, 29, 325–335. [Google Scholar] [CrossRef]
- Ko, K.S.; Kong, I.C. Influence of incubation conditions on the nanoparticles toxicity based on seed germination and bacterial bioluminescence. J. Nanosci. Nanotechnol. 2017, 17, 2382–2389. [Google Scholar] [CrossRef]









| Type | Size of Particle or Crystallite (nm) | Surface Area (m2/g) | ||||
|---|---|---|---|---|---|---|
| MFR a | XRD b | SEM/TEM c | SA d | MFR a | BET e | |
| A | 10–30 | 26 | 10–30 (> 50) | 25.3 | 40–70 | 38.94 |
| B | 50–80 | 69 | 80–150 (> 200, 500–1000) | 1062 | 10 | 0.929 |
| Conditions | Size 1 (type A) | Size 2 (type B) | ||
|---|---|---|---|---|
| low conc. b | high conc. | low conc. | high conc. | |
| Average toxicity (%) (except for shoot growth) a | 43 ± 7.0 | 63 ± 3.0 | 19 ± 5.5 | 32 ± 5.7 |
| 53 ± 5.0 | 25 ± 5.6 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, I.C.; Ko, K.-S.; Koh, D.-C.; Chon, C.-M. Comparative Effects of Particle Sizes of Cobalt Nanoparticles to Nine Biological Activities. Int. J. Mol. Sci. 2020, 21, 6767. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186767
Kong IC, Ko K-S, Koh D-C, Chon C-M. Comparative Effects of Particle Sizes of Cobalt Nanoparticles to Nine Biological Activities. International Journal of Molecular Sciences. 2020; 21(18):6767. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186767
Chicago/Turabian StyleKong, In Chul, Kyung-Seok Ko, Dong-Chan Koh, and Chul-Min Chon. 2020. "Comparative Effects of Particle Sizes of Cobalt Nanoparticles to Nine Biological Activities" International Journal of Molecular Sciences 21, no. 18: 6767. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21186767