Hyperbaric Oxygen Treatment Following Mid-Cervical Spinal Cord Injury Preserves Diaphragm Muscle Function
Abstract
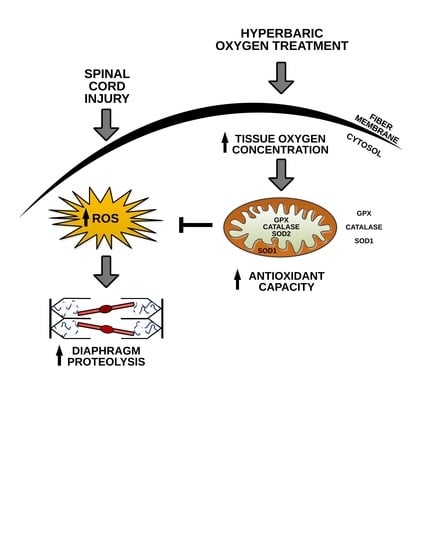
:1. Introduction
2. Results
2.1. Biological Response to Experimental Treatments
2.2. HBO Treatment after SCI Preserves Specific Force Production and Cross-Sectional Area
2.3. Mitochondrial Function Is Maintained Following SCI and HBO Treatment
2.4. HBO Therapy Upregulates Endogenous Antioxidant Expression in the Diaphragm
2.5. SCI Enhances Transcription of Inflammatory and Atrophy Markers in the Diaphragm
3. Discussion
3.1. HBO Therapy Attenuates Diaphragm Atrophy and Contractile Dysfunction after SCI
3.2. HBO Enhances Diaphragm Oxidative Capacity and Prevents Mitochondrial ROS Emission
3.3. Atrogene Expression Is Reduced in the Diaphragm Following SCI and HBO Treatment
4. Materials and Methods
4.1. Animals
4.1.1. Lateral-Cervical Spinal Cord Contusion
4.1.2. HBO Treatment
4.2. Functional Analysis
Diaphragm Contractile function
4.3. Histological Analysis
Diaphragm CSA
4.4. Biochemical Analysis
4.4.1. Permeabilized Muscle Fibers
4.4.2. Mitochondrial Respiration
4.4.3. ROS Production
4.4.4. Western Blotting
4.4.5. RNA Isolation and cDNA Synthesis
4.4.6. Real-Time Polymerase Chain Reaction
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, J.C.; Abdala, A.P.; Rybak, I.A.; Paton, J.F. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2577–2587. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, E.G.; Feldman, J.L. Brainstem network controlling descending drive to phrenic motoneurons in rat. J. Comp. Neurol. 1994, 347, 64–86. [Google Scholar] [CrossRef] [PubMed]
- Galeiras Vazquez, R.; Rascado Sedes, P.; Mourelo Farina, M.; Montoto Marques, A.; Ferreiro Velasco, M.E. Respiratory management in the patient with spinal cord injury. BioMed Res. Int. 2013, 2013, 168757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012, 50, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Smuder, A.J.; Gonzalez-Rothi, E.J.; Kwon, O.S.; Morton, A.B.; Sollanek, K.J.; Powers, S.K.; Fuller, D.D. Cervical spinal cord injury exacerbates ventilator-induced diaphragm dysfunction. J. Appl. Physiol. 2016, 120, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Redox control of skeletal muscle atrophy. Free Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Bhutani, S.; Vishwanath, G. Hyperbaric oxygen and wound healing. Indian J. Plast. Surg. 2012, 45, 316–324. [Google Scholar] [CrossRef]
- Moon, R.E. Hyperbaric oxygen treatment for decompression sickness. Undersea Hyperb. Med. 2014, 41, 151–157. [Google Scholar]
- Gregorevic, P.; Lynch, G.S.; Williams, D.A. Hyperbaric oxygen improves contractile function of regenerating rat skeletal muscle after myotoxic injury. J. Appl. Physiol. 2000, 89, 1477–1482. [Google Scholar] [CrossRef]
- Gregorevic, P.; Williams, D.A.; Lynch, G.S. Hyperbaric oxygen increases the contractile function of regenerating rat slow muscles. Med. Sci. Sports Exerc. 2002, 34, 630–636. [Google Scholar]
- Oyaizu, T.; Enomoto, M.; Yamamoto, N.; Tsuji, K.; Horie, M.; Muneta, T.; Sekiya, I.; Okawa, A.; Yagishita, K. Hyperbaric oxygen reduces inflammation, oxygenates injured muscle, and regenerates skeletal muscle via macrophage and satellite cell activation. Sci. Rep. 2018, 8, 1288. [Google Scholar] [CrossRef] [PubMed]
- Gregorevic, P.; Lynch, G.S.; Williams, D.A. Hyperbaric oxygen modulates antioxidant enzyme activity in rat skeletal muscles. Eur. J. Appl. Physiol. 2001, 86, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Gill, L.C.; Ross, H.H.; Lee, K.Z.; Gonzalez-Rothi, E.J.; Dougherty, B.J.; Judge, A.R.; Fuller, D.D. Rapid diaphragm atrophy following cervical spinal cord hemisection. Respir. Physiol. Neurobiol. 2014, 192, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef]
- Morton, A.B.; Mor Huertas, A.; Hinkley, J.M.; Ichinoseki-Sekine, N.; Christou, D.D.; Smuder, A.J. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion 2019, 45, 52–62. [Google Scholar] [CrossRef]
- Roberts, B.M.; Ahn, B.; Smuder, A.J.; Al-Rajhi, M.; Gill, L.C.; Beharry, A.W.; Powers, S.K.; Fuller, D.D.; Ferreira, L.F.; Judge, A.R. Diaphragm and ventilatory dysfunction during cancer cachexia. FASEB J. 2013, 27, 2600–2610. [Google Scholar] [CrossRef] [Green Version]
- Nicaise, C.; Hala, T.J.; Frank, D.M.; Parker, J.L.; Authelet, M.; Leroy, K.; Brion, J.P.; Wright, M.C.; Lepore, A.C. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp. Neurol. 2012, 235, 539–552. [Google Scholar] [CrossRef]
- Rana, S.; Sieck, G.C.; Mantilla, C.B. Diaphragm electromyographic activity following unilateral midcervical contusion injury in rats. J. Neurophysiol. 2017, 117, 545–555. [Google Scholar] [CrossRef]
- Mantilla, C.B.; Greising, S.M.; Zhan, W.Z.; Seven, Y.B.; Sieck, G.C. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J. Appl. Physiol. 2013, 114, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Zhan, W.Z.; Miyata, H.; Prakash, Y.S.; Sieck, G.C. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J. Appl. Physiol. 1997, 82, 1145–1153. [Google Scholar] [CrossRef]
- Alvarez-Argote, S.; Gransee, H.M.; Mora, J.C.; Stowe, J.M.; Jorgenson, A.J.; Sieck, G.C.; Mantilla, C.B. The impact of midcervical contusion injury on diaphragm muscle function. J. Neurotrauma 2016, 33, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicaise, C.; Frank, D.M.; Hala, T.J.; Authelet, M.; Pochet, R.; Adriaens, D.; Brion, J.P.; Wright, M.C.; Lepore, A.C. Early phrenic motor neuron loss and transient respiratory abnormalities after unilateral cervical spinal cord contusion. J. Neurotrauma 2013, 30, 1092–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicaise, C.; Putatunda, R.; Hala, T.J.; Regan, K.A.; Frank, D.M.; Brion, J.P.; Leroy, K.; Pochet, R.; Wright, M.C.; Lepore, A.C. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J. Neurotrauma 2012, 29, 2748–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhu, M.S.; Dougherty, B.J.; Lane, M.A.; Bolser, D.C.; Kirkwood, P.A.; Reier, P.J.; Fuller, D.D. Respiratory recovery following high cervical hemisection. Respir. Physiol. Neurobiol. 2009, 169, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Sayir, F.; Kavak, S.; Meral, I.; Demir, H.; Cengiz, N.; Cobanoglu, U. Effects of crush and axotomy on oxidative stress and some trace element levels in phrenic nerve of rats. Brain Res. Bull. 2013, 92, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Gorgey, A.S.; Witt, O.; O’Brien, L.; Cardozo, C.; Chen, Q.; Lesnefsky, E.J.; Graham, Z.A. Mitochondrial health and muscle plasticity after spinal cord injury. Eur. J. Appl. Physiol. 2019, 119, 315–331. [Google Scholar] [CrossRef]
- Mancinelli, R.; Kern, H.; Fulle, S.; Carraro, U.; Zampieri, S.; La Rovere, R.; Fano, G.; Pietrangelo, T. Transcriptional profile of denervated vastus lateralis muscle derived from a patient 8 months after spinal cord injury: A case-report. Int. J. Immunopathol. Pharmacol. 2011, 24, 749–759. [Google Scholar] [CrossRef]
- Shah, P.K.; Ye, F.; Liu, M.; Jayaraman, A.; Baligand, C.; Walter, G.; Vandenborne, K. In vivo (31)P NMR spectroscopy assessment of skeletal muscle bioenergetics after spinal cord contusion in rats. Eur. J. Appl. Physiol. 2014, 114, 847–858. [Google Scholar] [CrossRef]
- Gregory, C.M.; Vandenborne, K.; Castro, M.J.; Dudley, G.A. Human and rat skeletal muscle adaptations to spinal cord injury. Can. J. Appl. Physiol. 2003, 28, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Makowski, N.S.; Lombardo, L.M.; Foglyano, K.M.; Kobetic, R.; Pinault, G.; Selkirk, S.M.; Triolo, R.J. Walking after incomplete spinal cord injury with an implanted neuromuscular electrical stimulation system and a hinged knee replacement: A single-subject study. Spinal Cord Ser. Cases 2020, 6, 86. [Google Scholar] [CrossRef]
- Mancinelli, R.; Toniolo, L.; Di Filippo, E.S.; Doria, C.; Marrone, M.; Maroni, C.R.; Verratti, V.; Bondi, D.; Maccatrozzo, L.; Pietrangelo, T.; et al. Neuromuscular electrical stimulation induces skeletal muscle fiber remodeling and specific gene expression profile in healthy elderly. Front. Physiol. 2019, 10, 1459. [Google Scholar] [CrossRef] [Green Version]
- Smuder, A.J.; Morton, A.B.; Hall, S.E.; Wiggs, M.P.; Ahn, B.; Wawrzyniak, N.R.; Sollanek, K.J.; Min, K.; Kwon, O.S.; Nelson, W.B.; et al. Effects of exercise preconditioning and HSP72 on diaphragm muscle function during mechanical ventilation. J. Cachexia Sarcopenia Muscle 2019, 10, 767–781. [Google Scholar] [CrossRef] [Green Version]
- Godman, C.A.; Joshi, R.; Giardina, C.; Perdrizet, G.; Hightower, L.E. Hyperbaric oxygen treatment induces antioxidant gene expression. Ann. N. Y. Acad. Sci. 2010, 1197, 178–183. [Google Scholar] [CrossRef]
- Rothfuss, A.; Speit, G. Investigations on the mechanism of hyperbaric oxygen (HBO)-induced adaptive protection against oxidative stress. Mutat. Res. 2002, 508, 157–165. [Google Scholar] [CrossRef]
- Barata, P.; Cervaens, M.; Resende, R.; Camacho, O.; Marques, F. Hyperbaric oxygen effects on sports injuries. Ther. Adv. Musculoskelet. Dis. 2011, 3, 111–121. [Google Scholar] [CrossRef]
- Urso, M.L.; Chen, Y.W.; Scrimgeour, A.G.; Lee, P.C.; Lee, K.F.; Clarkson, P.M. Alterations in mRNA expression and protein products following spinal cord injury in humans. J. Physiol. 2007, 579 Pt 3, 877–892. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Hyatt, J.P.; Raffaello, A.; Jagoe, R.T.; Roy, R.R.; Edgerton, V.R.; Lecker, S.H.; Goldberg, A.L. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007, 21, 140–155. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Gomes, A.V.; Waddell, D.S.; Siu, R.; Stein, M.; Dewey, S.; Furlow, J.D.; Bodine, S.C. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB J. 2012, 26, 2986–2999. [Google Scholar] [CrossRef] [Green Version]
- Dodd, S.L.; Gagnon, B.J.; Senf, S.M.; Hain, B.A.; Judge, A.R. Ros-mediated activation of NF-kappaB and Foxo during muscle disuse. Muscle Nerve 2010, 41, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Smuder, A.J.; Hudson, M.B.; Nelson, W.B.; Kavazis, A.N.; Powers, S.K. Nuclear factor-kappaB signaling contributes to mechanical ventilation-induced diaphragm weakness*. Crit. Care Med. 2012, 40, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, M.A.; Lee, K.Z.; Salazar, K.; O’Steen, B.E.; Bloom, D.C.; Fuller, D.D.; Reier, P.J. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp. Neurol. 2012, 235, 197–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, S.K.; Shanely, R.A.; Coombes, J.S.; Koesterer, T.J.; McKenzie, M.; Van Gammeren, D.; Cicale, M.; Dodd, S.L. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J. Appl. Physiol. 2002, 92, 1851–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavazis, A.N.; Talbert, E.E.; Smuder, A.J.; Hudson, M.B.; Nelson, W.B.; Powers, S.K. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic. Biol. Med. 2009, 46, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Morton, A.B.; Smuder, A.J.; Wiggs, M.P.; Hall, S.E.; Ahn, B.; Hinkley, J.M.; Ichinoseki-Sekine, N.; Huertas, A.M.; Ozdemir, M.; Yoshihara, T.; et al. Increased SOD2 in the diaphragm contributes to exercise-induced protection against ventilator-induced diaphragm dysfunction. Redox Biol. 2018, 20, 402–413. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Gao, C.; Li, Z.; Yang, J.; Liu, X.; Liang, F. Effects of hyperbaric oxygen therapy on RAGE and MCP-1 expression in rats with spinal cord injury. Mol. Med. Rep. 2016, 14, 5619–5625. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, D.; Su, P.; Lin, F.; Tang, Q. Changes in autophagy in rats after spinal cord injury and the effect of hyperbaric oxygen on autophagy. Neurosci. Lett. 2016, 618, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, S.; Luo, M.; Li, Y. Hyperbaric oxygen therapy improves local microenvironment after spinal cord injury. Neural Regen. Res. 2014, 9, 2182–2188. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smuder, A.J.; Turner, S.M.; Schuster, C.M.; Morton, A.B.; Hinkley, J.M.; Fuller, D.D. Hyperbaric Oxygen Treatment Following Mid-Cervical Spinal Cord Injury Preserves Diaphragm Muscle Function. Int. J. Mol. Sci. 2020, 21, 7219. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197219
Smuder AJ, Turner SM, Schuster CM, Morton AB, Hinkley JM, Fuller DD. Hyperbaric Oxygen Treatment Following Mid-Cervical Spinal Cord Injury Preserves Diaphragm Muscle Function. International Journal of Molecular Sciences. 2020; 21(19):7219. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197219
Chicago/Turabian StyleSmuder, Ashley J., Sara M. Turner, Cassandra M. Schuster, Aaron B. Morton, J. Matthew Hinkley, and David D. Fuller. 2020. "Hyperbaric Oxygen Treatment Following Mid-Cervical Spinal Cord Injury Preserves Diaphragm Muscle Function" International Journal of Molecular Sciences 21, no. 19: 7219. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197219