The Paralogue of the Intrinsically Disordered Nuclear Protein 1 Has a Nuclear Localization Sequence that Binds to Human Importin α3
Abstract
:1. Introduction
2. Results
2.1. Intact NUPR1L is Associated with Both Impα3 and ∆Impα3
2.2. Isolated NLS-NUPR1L Was Bound to Impα3 and ∆Impα3
2.2.1. Isolated NLS-NUPR1L Was Monomeric and Disordered in Solution
2.2.2. Isolated NLS-NUPR1L Associated with Both Importins
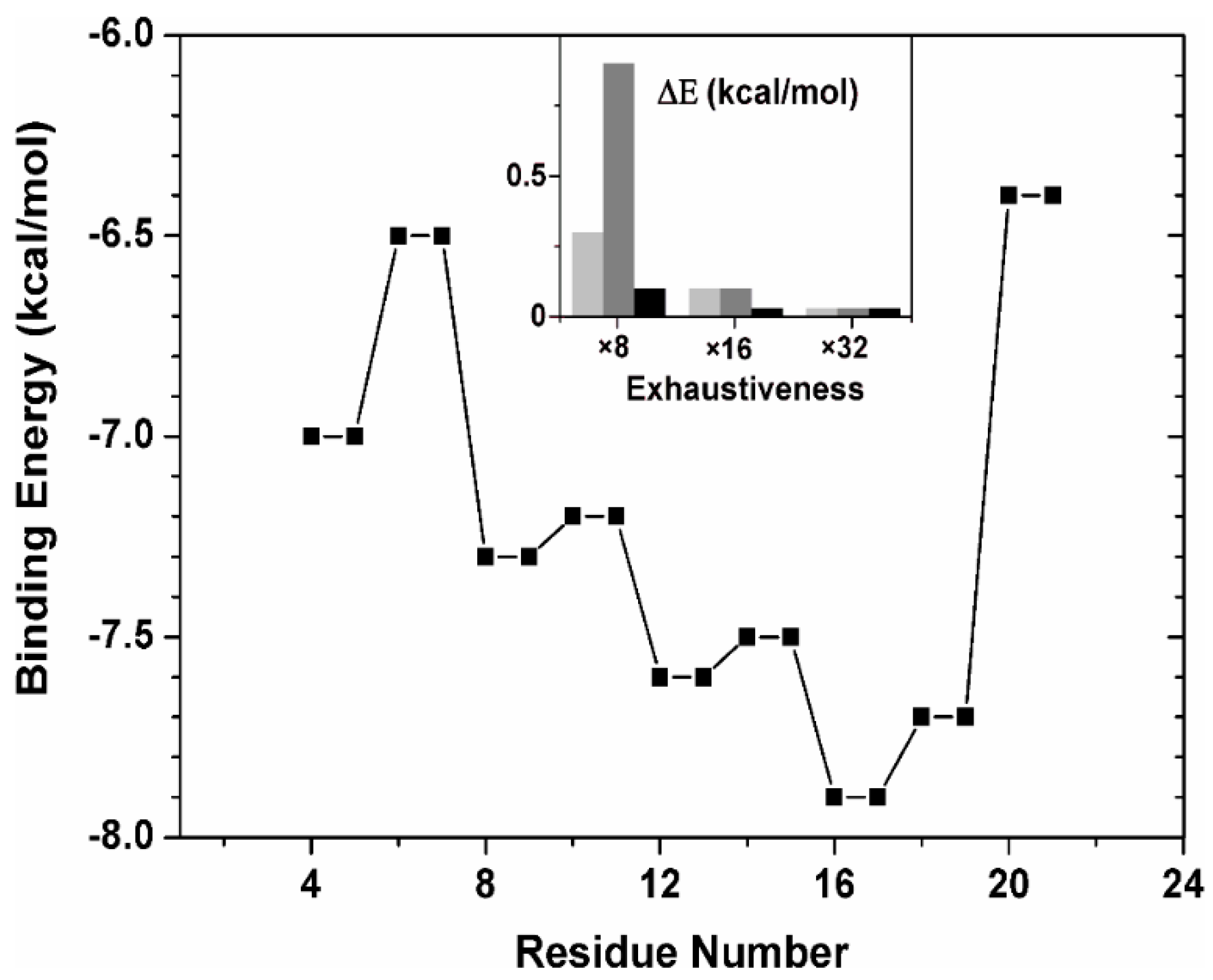
2.2.3. Binding Regions in the Docking of NLS-NUPR1L to Importins
3. Discussion
3.1. Identification of the NLS Region of NUPR1L
3.2. The Inhibitory Effect of the IBB in Impα3
3.3. Binding to Impα3 of NUPR1L
4. Materials and Methods
4.1. Materials
4.2. Protein Expression and Purification
4.3. Prediction and Synthesis of NLS-NUPR1L
4.4. Fluorescence
4.4.1. Steady-State Fluorescence
4.4.2. Binding Experiments
4.5. CD
4.5.1. Far-UV CD Spectra
4.5.2. Thermal Denaturations
4.6. ITC
4.7. NMR
4.7.1. D-1H-NMR Spectrum
4.7.2. Translational Diffusion NMR (DOSY)
4.7.3. D-1H-NMR Spectroscopy
4.7.4. Measurements of T2
4.8. Molecular Docking
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ARM | Armadillo |
| CD | Circular dichroism |
| DOSY | Diffusion ordered spectroscopy |
| DIPSI | Decoupling in the presence of scalar interactions |
| IBB | Importin β-binding domain |
| IDP | Intrinsically disordered protein |
| Impα3 | Human importin α3 isoform (residues 1–521) |
| ∆Impα3 | Truncated species of Impα3 (residues 64-521) depleted of the IBB |
| ITC | Isothermal titration calorimetry |
| NLS | Nuclear localization sequence |
| NLS-NUPR1L | Nuclear localization sequence of NUPR1L (residues 51–74) |
| NOE | Nuclear Overhauser effect |
| NOESY | Nuclear Overhauser effect spectroscopy |
| NPC | Nuclear pore complex |
| NUPR1 | Nuclear protein 1 |
| NUPR1L | The NUPR1-like paralogue |
| TOCSY | Total correlation spectroscopy |
| TPPI | Time-proportional-phase incrementation technique |
| UV | Ultraviolet |
References
- Mallo, G.V.; Fiedler, F.; Calvo, E.L.; Ortiz, E.M.; Vasseur, S.; Keim, V.; Morisset, J.; Iovanna, J.L. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J. Biol. Chem. 1997, 72, 32360–32369. [Google Scholar] [CrossRef] [Green Version]
- Chowdury, U.R.; Samant, R.S.; Fodstat, O.; Shevde, L.A. Emerging role of nuclear protein 1 (nupr1) in cancer biology. Cancer Metastasis Rev. 2009, 28, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. A decade and a half of protein intrinsic disorder: Biology still waits for physics. Protein Sci. 2013, 22, 693–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlow, R.B.; Dyson, H.J.; Wright, P.E. Expanding the paradigm: Intrinsically disordered proteins and allosterism. J. Mol. Biol. 2018, 430, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.E.; Dyson, H.J. Intrinsically disordered proteins in cellular signalling and regulation. Nat. Mol. Cell Biol. 2015, 16, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Cano, C.E.; Hamidi, T.; Sandi, M.J.; Iovanna, J.L. Nupr-1: The Swiss knife of cancer. J. Cell Physiol. 2011, 226, 1439–1443. [Google Scholar] [CrossRef]
- Goruppi, S.; Iovanna, J.L. Stress-inducible protein p8 is involved in several physiological and pathological processes. J. Biol. Chem. 2010, 285, 1577–1581. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, T.; Algül, H.; Cano, C.E.; Sandi, M.J.; Molejon, M.I.; Riemann, M.; Calvo, E.L.; Lomberk, G.; Dagorn, J.C.; Weih, F.; et al. Nuclear protein 1 promotes pancreatic cancer development and protects cells from stress by inhibiting apoptosis. J. Clin. Investig. 2012, 122, 2092–2103. [Google Scholar] [CrossRef] [Green Version]
- Malicet, C.; Giroux, V.; Vasseur, S.; Dagorn, J.C.; Neira, J.L.; Iovanna, J.L. Regulation of apoptosis by the p8/prothymosin alpha complex. Proc. Natl. Acad. Sci. USA 2006, 103, 2671–2676. [Google Scholar] [CrossRef] [Green Version]
- Encinar, J.A.; Mallo, G.V.; Mizyrycki, C.; Giono, L.; González-Ros, J.M.; Rico, M.; Cánepa, E.; Moreno, S.; Neira, J.L.; Iovanna, J.L. Human p8 is a HMG-I/Y-like protein with DNA binding activity enhanced by phosphorylation. J. Biol. Chem. 2001, 276, 2742–2751. [Google Scholar] [CrossRef] [Green Version]
- Aguado-Llera, D.; Hamidi, T.; Doménech, R.; Pantoja-Uceda, D.; Gironella, M.; Santoro, J.; Velázquez-Campoy, A.; Neira, J.L.; Iovanna, J.L. Deciphering the binding between Nupr1 and MSL1 and their DNA-repairing activity. PLoS ONE 2013, 8, e78101. [Google Scholar] [CrossRef]
- Santofimia-Castaño, P.; Rizzuti, B.; Pey, A.L.; Soubeyran, P.; Vidal, M.; Urrutia, R.; Iovanna, J.L.; Neira, J.L. Intrinsically disordered chromatin protein NUPR1 binds to the C-terminal region of Polycomb RING1B. Proc. Natl. Acad. Sci. USA 2017, 114, 6332–6341. [Google Scholar]
- López, M.B.; García, M.N.; Grasso, D.; Bintz, J.; Molejón, M.I.; Vélez, G.; Lomberk, G.; Neira, J.L.; Urrutia, R.; Iovanna, J.L. Functional characterization of NUPR1L, a novel p53-regulated isoform of the high-mobility group (HMG)-related protumoral protein NUPR1. J. Cell. Physiol. 2015, 230, 2936–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neira, J.L.; López, M.B.; Sevilla, P.; Rizzuti, B.; Cámara-Artigas, A.; Vidal, M.; Iovanna, J.L. The chromatin nuclear protein NUPR1L is intrinsically disordered and binds to the same proteins as its paralogue. Biochem. J. 2018, 475, 2271–2291. [Google Scholar] [CrossRef]
- Stewart, M. Molecular mechanism of the nuclear protein import cycle. Nat. Rev. Mol. Cell. Biol. 2007, 8, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Bednenko, J.; Cingolari, G.; Gerace, L. Nucleo-cytoplasmic transport navigating the channel. Traffic 2003, 4, 127–135. [Google Scholar] [CrossRef]
- Cingolani, G.; Bednenko, J.; Gillespie, M.T.; Gerace, L. Molecular basis for the recognition of a non-classical nuclear localization signal by importin beta. Mol. Cell 2002, 10, 1345–1353. [Google Scholar] [CrossRef]
- Goldfarb, D.S.; Corbett, A.H.; Mason, D.A.; Harreman, M.T.; Adam, S.A. Importin: A multipurpose nuclear-transport receptor. Trends Cell Biol. 2004, 14, 505–514. [Google Scholar] [CrossRef]
- Pumroy, R.A.; Cingolani, G. Diversification of importin-alpha isoforms in cellular trafficking and disease states. Biochem. J. 2015, 466, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Loveland, K.L.; Yoneda, Y. Nuclear importin α and its physiological importance. Comm. Integ. Biol. 2012, 5, 220–222. [Google Scholar] [CrossRef]
- Marvaldi, L.; Panayotokis, N.; Alber, S.; Dgan, S.Y.; Okladnikov, N.; Koppel, I.; Di Pizio, A.; Song, D.-A.; Tzur, Y.; Terenzio, M.; et al. Importin 3 regulates chronic pain pathways in peripheral sensory neurons. Science 2020, 369, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Kobe, B. Autoinhibition by an internal nuclear localization signal revealed by the crystal structure of mammalian importin. Nat. Struct. Biol. 1999, 6, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Santofimia-Castaño, P.; Swayden, M.; Xia, Y.; Zhou, Z.; Audebert, S.; Camoin, L.; Huang, C.; Peng, L.; Jiménez-Alesanco, A.; et al. ZZW-115-dependent inhibition of NUPR1 nuclear translocation sensitizes cancer cells to genotoxic agents. JCI Insight 2020, 138117. [Google Scholar] [CrossRef] [PubMed]
- Valacco, M.P.; Varone, C.; Malicet, C.; Cánepa, E.; Iovanna, J.L.; Moreno, S. Cell growth-dependent subcellular localization of p8. J. Cell Biochem. 2006, 97, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, C.; Hornos, F.; Giudici, A.M.; Cámara-Artigas, A.; Luque-Ortega, J.L.; Arbe, A.; Rizzuti, B.; Alfonso, C.; Forwood, J.K.; Iovanna, J.L.; et al. Human importin α3 and its N-terminal truncated form, without the importin-β-binding domain, are oligomeric species with a low conformational stability in solution. BBA Gen. Subj. 2020, 1864, 129609. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Scholtz, J.M. Measuring the Conformational Stability of A Protein. In Protein Structure, 2nd ed.; Creighton, T.E., Ed.; Oxford University Press: Oxford, UK, 1997; pp. 253–259. [Google Scholar]
- Wüthrich, K. NMR of Proteins and Nucleic Acids; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Danielsson, J.; Jarvet, J.; Damberg, P.; Gräslund, A. Translational diffusion measured by PFG-NMR on full length and fragments of the Alzheimer Aβ (1-40) peptide. Determination of hydrodynamic radii of random coil peptides of varying length. Magn. Reson. Chem. 2002, 40, S89–S97. [Google Scholar] [CrossRef]
- Kjaergaard, M.; Brander, S.; Poulsen, F.M. Random coil chemical shifts for intrinsically disordered proteins: Effects of temperature and pH. J. Biomol. NMR 2011, 49, 139–149. [Google Scholar] [CrossRef]
- Kjaergaard, M.; Poulsen, F.M. Sequence correction of random coil chemical shifts: Correlation between neighbour correction factors and changes in the Ramachandran distribution. J. Biomol. NMR 2011, 50, 157–165. [Google Scholar] [CrossRef]
- Cavanagh, J.; Fairbrother, W.J.; Palmer, A.G.; Skelton, N.J. Protein NMR Spectroscopy: Principles and Practice; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Neira, J.L.; Hornos, F.; Cozza, C.; Cámara-Artigas, A.; Abián, O.; Velázquez-Campoy, A. The histidine phosphocarrier protein, HPr, binds to the highly thermostable regulator of sigma D protein, Rsd, and its isolated helical fragments. Arch. Biochem. Biophys. 2018, 639, 26–37. [Google Scholar] [CrossRef]
- Cremades, N.; Velázquez-Campoy, A.; Freire, E.; Sancho, J. The flavodoxin from Helicobacter pylori: Structural determinants of thermostability and FMN cofactor binding. Biochemistry 2008, 47, 627–639. [Google Scholar] [CrossRef] [Green Version]
- Bollen, Y.J.; Westphal, A.H.; Lindhoud, S.; van Berkel, W.J.; van Mierlo, C.P. Distant residues mediate picomolar binding affinity of a protein cofactor. Nat. Commun. 2012, 3, 1010. [Google Scholar] [CrossRef] [PubMed]
- Yadahalli, S.; Neira, J.L.; Johnson, C.M.; Tan, Y.S.; Rowling, P.J.E.; Chattopadhyay, A.; Verma, C.S.; Itzhaki, L.S. Kinetic and thermodynamic effects of phosphorylation on p53 binding to MDM2. Sci. Rep. 2019, 24, 693. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakada, R.; Matsuura, Y. Crystal structure of importin-alpha bound to the nuclear localization signal of Epstein-Barr virus EBNA-LP protein. Protein Sci. 2017, 26, 1231–1235. [Google Scholar] [CrossRef] [Green Version]
- Delano, W.L. Available online: http://www.pymol.org/ (accessed on 5 October 2002).
- Junod, S.L.; Kelich, J.M.; Ma, J.; Yang, W. Nucleocytoplasmic transport of intrinsically disordered proteins studies by high-speed super-resolution microscopy. Protein Sci. 2020, 29, 1459–1472. [Google Scholar] [CrossRef]
- Smith, K.M.; Tsimbalyuk, S.; Edwards, M.G.; Cross, E.M.; Batra, J.; Soares da Costa, T.P.; Aragao, D.; Basler, C.F.; Forwood, J.K. Structural basis for importin alpha 3 specificity of W proteins in Hendra and Nipah viruses. Nat. Commun. 2018, 9, 3703. [Google Scholar] [CrossRef]
- Neira, J.L.; Rizzuti, B.; Jiménez-Alesanco, A.; Palomino-Schätzlein, M.; Abián, O.; Velázquez-Campoy, A.; Iovanna, J.L. A Phosphorylation-Induced Switch in the Nuclear Localization Sequence of the Intrinsically Disordered NUPR1 Hampers Binding to Importin. Biomolecules 2020, 10, 1313. [Google Scholar] [CrossRef]
- Miyatake, H.; Sanjoh, A.; Unzai, S.; Mtsuda, G.; Tatsumi, Y.; Miyamoto, Y.; Dohmae, N.; Aida, Y. Crystal structure of human importin α1 (Rch1) revealing a potential autoinhibition mode involving homodimerization. PLoS ONE 2015, 20, e0115995. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of yeast cell cycle-dependent nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef] [Green Version]
- Kosugi, S.; Hasebe, M.; Matsumura, N.; Takashima, H.; Miyamoto-Sato, E.; Tomita, M.; Yanagawa, H. Six classes of nuclear localization signals specific to different binding grooves of importin α. J. Biol. Chem. 2009, 284, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Neira, J.L.; Hornos, F.; Bacarizo, J.; Cámara-Artigas, A.; Gómez, J. The monomeric species of the regulatory domain of tyrosine hydroxylase has a low conformational stability. Biochemistry 2017, 55, 3418–3431. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, B.; King, R.W.; Wheeler, M.R.; Lewis, C.A., Jr.; Goode, S.; Dunlap, R.B.; Roberts, G.C. Correction for light absorption in fluorescence studies of protein-ligand interactions. Anal. Biochem. 1983, 132, 353–361. [Google Scholar] [CrossRef]
- Beckett, D. Measurement and analysis of equilibrium binding titrations: A beginner’s guide. Methods Enzymol. 2011, 488, 1–16. [Google Scholar] [PubMed]
- Royer, C.A.; Scarlatta, S.F. Fluorescence approaches to quantifying biomolecular interactions. Methods Enzymol. 2008, 450, 79–106. [Google Scholar] [PubMed]
- Benjwal, S.; Verma, S.; Röhm, K.H.; Gursky, O. Monitoring protein aggregation during thermal unfolding in circular dichroism experiments. Protein Sci. 2006, 15, 635–639. [Google Scholar] [CrossRef]
- Santofimia-Castaño, P.; Xia, Y.; Lan, W.; Zhou, Z.; Huang, C.; Peng, L.; Soubeyran, P.; Velázquez-Campoy, A.; Abian, O.; Rizzuti, B.; et al. Ligand-based design identifies a potent NUPR1 inhibitor exerting anticancer activity via necroptosis. J. Clin. Investig. 2019, 129, 2500–2513. [Google Scholar]
- Piotto, M.; Saudek, V.; Sklenar, V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR 1992, 2, 661–675. [Google Scholar] [CrossRef]
- Wilkins, D.K.; Grimshaw, S.B.; Receveur, V.; Dobson, C.M.; Jones, J.A.; Smith, L.J. Hydrodynamic radii of native and denatured proteins measured by pulse field gradient NMR technique. Biochemistry 1999, 38, 16424–16431. [Google Scholar] [CrossRef]
- Marion, D.; Wüthrich, K. Application of phase sensitive two-dimensional correlated spectroscopy (COSY) for measurements of 1H-1H spin-spin coupling constants in proteins. Biochem. Biophys. Res. Commun. 1983, 11, 967–975. [Google Scholar] [CrossRef]
- Bax, A.; Davis, D.G. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J. Magn. Reson. 1985, 65, 355–360. [Google Scholar] [CrossRef]
- Kumar, A.; Ernst, R.R.; Wüthrich, K. A two-dimensional nuclear Overhauser enhancement (2D NOE) experiment for the elucidation of complete proton-proton cross-relaxation networks in biological macromolecules. Biochem. Biophys. Res. Commun. 1980, 95, 1–6. [Google Scholar] [CrossRef]
- Cavanagh, J.; Rance, M. Suppression of cross-relaxation effects in TOCSY spectra via a modified DIPSI-2 mixing sequence. J. Magn. Reson. 1992, 96, 660–678. [Google Scholar] [CrossRef]
- Anglister, J.; Grzesiek, S.; Ren, H.; Klee, C.B.; Bax, A. Isotope-edited multidimensional NMR of calcineurin B in the presence of the non-deuterated detergent CHAPS. J. Biomol. NMR 1993, 3, 121–126. [Google Scholar] [CrossRef]
- Sklenar, V.; Bax, A. Spin echo water suppression for the generation of pure-phase two-dimensional NMR spectra. J. Magn. Reson. 1987, 74, 469–479. [Google Scholar] [CrossRef]
- Neira, J.L.; Correa, J.; Rizzuti, B.; Santofimia-Castaño, P.; Abian, O.; Velázquez-Campoy, A.; Fernández-Megía, E.; Iovanna, J.L. Dendrimers as competitors of protein-protein interactions of the intrinsically disordered nuclear chromatin protein NUPR1. Biomacromolecules 2019, 20, 2567–2576. [Google Scholar] [CrossRef]
- Grande, F.; Rizzuti, B.; Occhiuzzi, M.A.; Ioele, G.; Casacchia, T.; Gelmini, F.; Guzzi, R.; Garofalo, A.; Statti, G. Identification by molecular docking of homoisoflavones from Leopoldia comosa as ligands of estrogen receptors. Molecules 2018, 23, 894. [Google Scholar] [CrossRef] [Green Version]



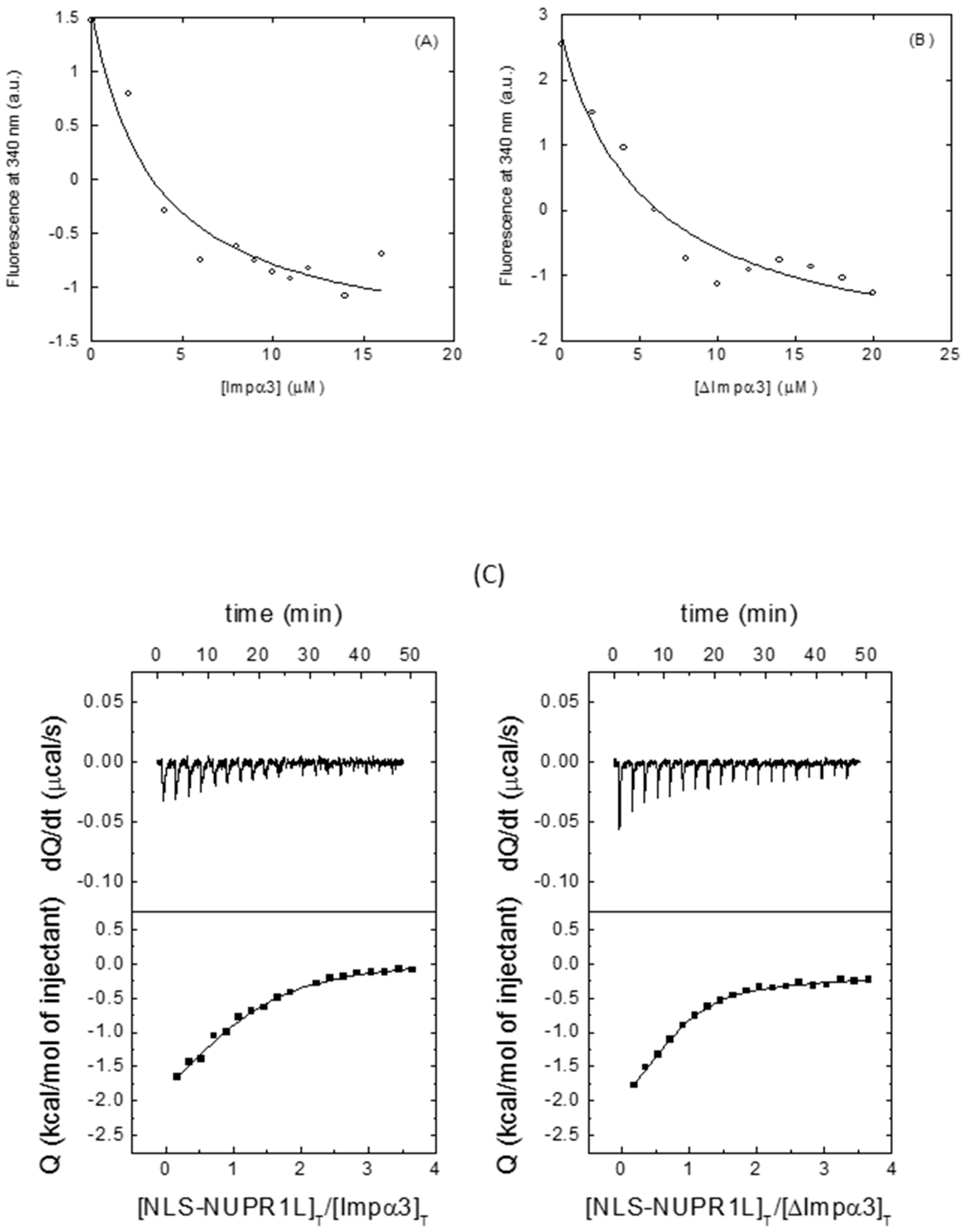

| Fluorescence | ITC | ||||
|---|---|---|---|---|---|
| Importin Species | Kd (μM) | Kd (μM) | ∆H (kcal mol−1) | n | |
| Impα3 | 3 ± 1 | 12 ± 2 | −3.1 ± 0.5 | 1.04 ± 0.05 | |
| ∆Impα3 | 5 ± 2 | 5.5 ± 0.9 | −2.4 ± 0.5 | 0.75 ± 0.06 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neira, J.L.; Rizzuti, B.; Jiménez-Alesanco, A.; Abián, O.; Velázquez-Campoy, A.; Iovanna, J.L. The Paralogue of the Intrinsically Disordered Nuclear Protein 1 Has a Nuclear Localization Sequence that Binds to Human Importin α3. Int. J. Mol. Sci. 2020, 21, 7428. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197428
Neira JL, Rizzuti B, Jiménez-Alesanco A, Abián O, Velázquez-Campoy A, Iovanna JL. The Paralogue of the Intrinsically Disordered Nuclear Protein 1 Has a Nuclear Localization Sequence that Binds to Human Importin α3. International Journal of Molecular Sciences. 2020; 21(19):7428. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197428
Chicago/Turabian StyleNeira, José L., Bruno Rizzuti, Ana Jiménez-Alesanco, Olga Abián, Adrián Velázquez-Campoy, and Juan L. Iovanna. 2020. "The Paralogue of the Intrinsically Disordered Nuclear Protein 1 Has a Nuclear Localization Sequence that Binds to Human Importin α3" International Journal of Molecular Sciences 21, no. 19: 7428. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21197428







