Conflicting Actions of Inhalational Anesthetics, Neurotoxicity and Neuroprotection, Mediated by the Unfolded Protein Response
Abstract
:1. Introduction
2. Results
2.1. Exposure to Inhalational Anesthetics Induces the UPR
2.2. Anesthetic Exposure Caused Cognitive Impairment in the Developing Brain
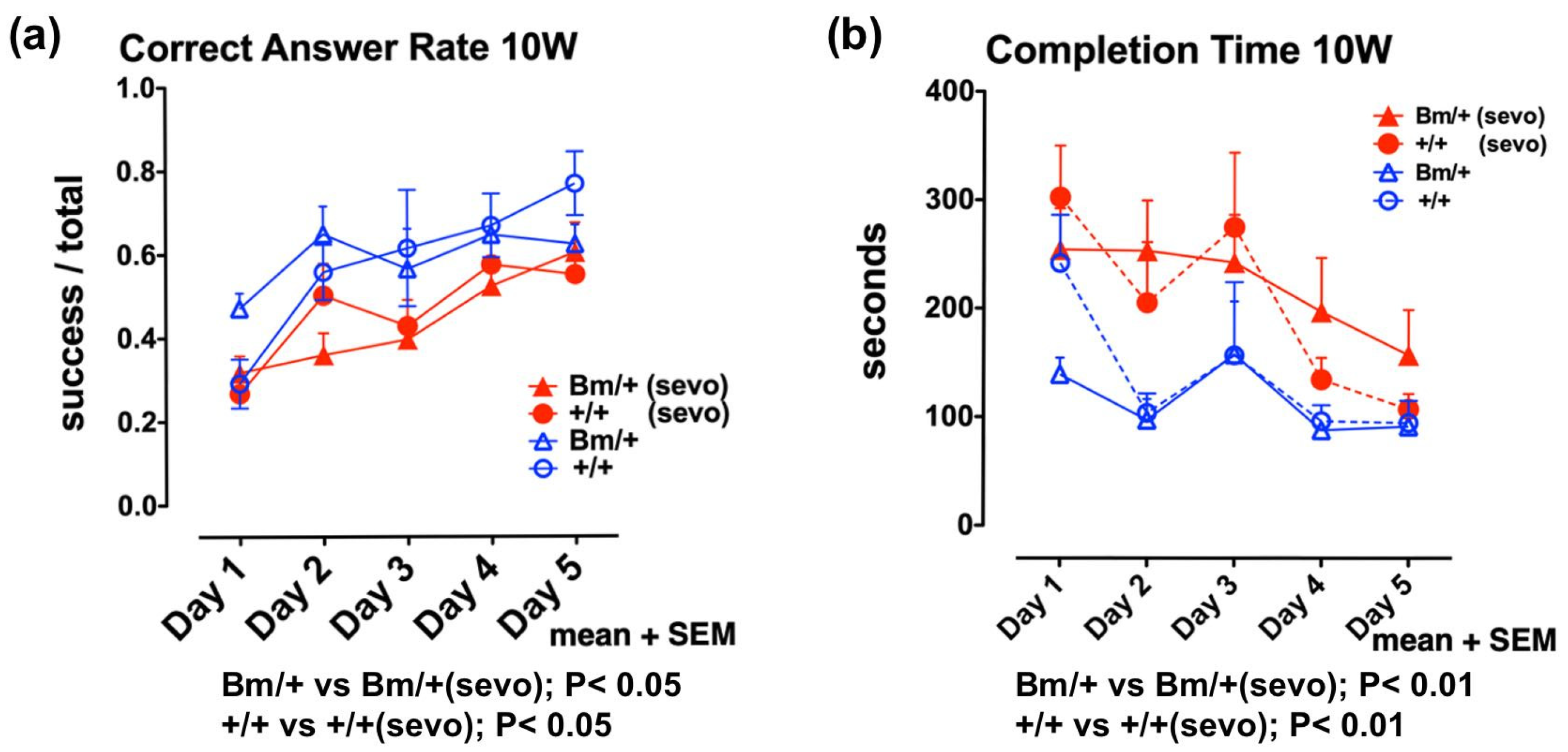
2.3. Moderate Exposure to Anesthetics Causes Cytoprotective Effects through the UPR
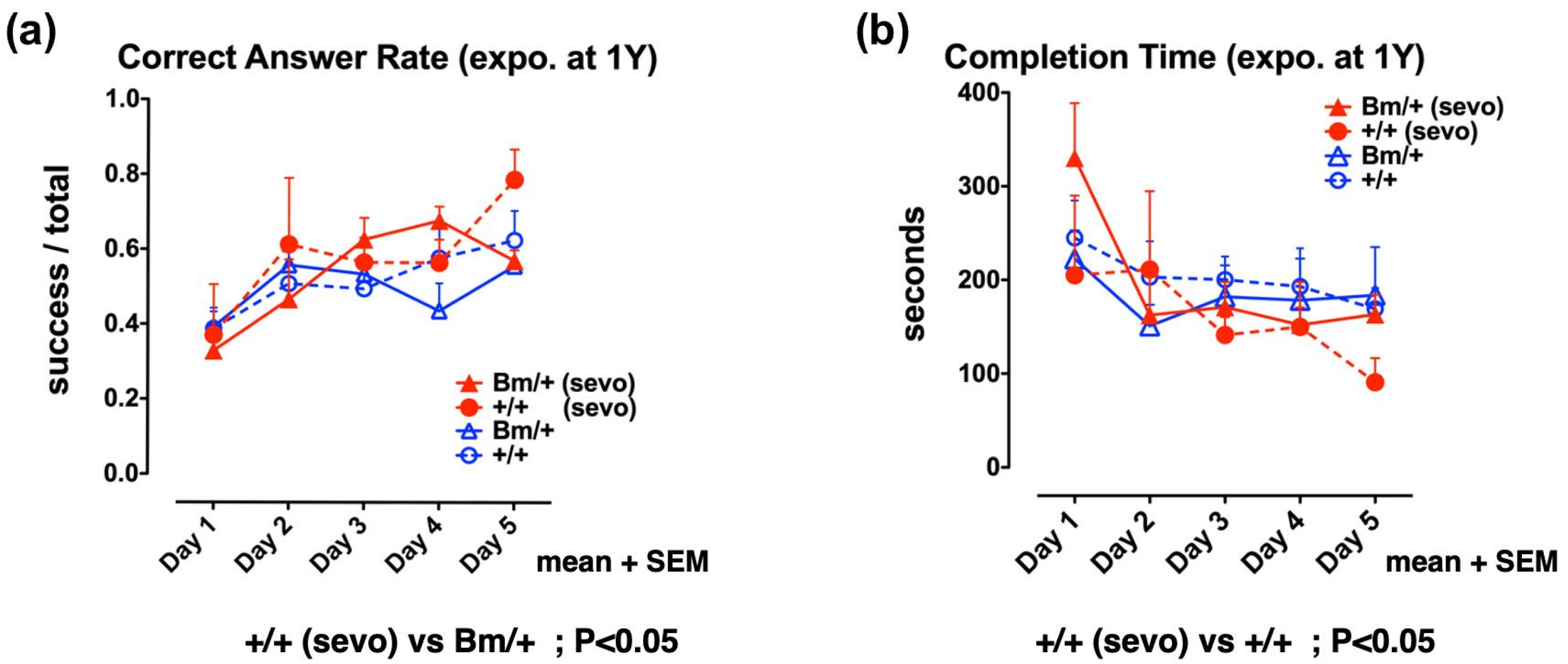
2.4. Pre-Exposure to Sevoflurane Improves Cognitive Function in Adult Wild-Type Mice, but not in Bip Mutant Mice
3. Discussion
4. Materials and Methods
4.1. Cells and Reagents
4.2. Animals
4.3. Inhalational Anesthetic Exposure
4.4. Eight-Arm Radial Maze Test
4.5. Western Blotting
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ER | Endoplasmic reticulum |
| UPR | Unfolded protein response |
| BiP | Binding immunoglobulin protein |
| KDEL | Lys-Asp-Glu-Leu |
| COPI | Coat protein I |
| CHOP | C/EBP homologous protein |
| PERK | Protein kinase RNA (PKR)-like ER kinase |
| IRE1 | Inositol requiring enzyme 1 |
| ATF6 | Activating transcription factor 6 |
| XBP1 | X-box-binding protein 1 |
| NMDA | N-methyl-D-aspartate |
| ITPR | Inositol 1,4,5-triphosphate receptor |
| MAC | Minimum alveolar concentration |
| MCI | Mild cognitive impairment |
References
- Jevtovic-Todorovic, V.; Hartman, R.E.; Izumi, Y.; Benshoff, N.D.; Dikranian, K.; Zorumski, C.F.; Olney, J.W.; Wozniak, D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003, 23, 876–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jevtovic-Todorovic, V. General Anesthetics and Neurotoxicity: How Much Do We Know? Anesthesiol. Clin. 2016, 34, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Stratmann, G.; Sall, J.W.; May, L.D.; Bell, J.S.; Magnusson, K.R.; Rau, V.; Visrodia, K.H.; Alvi, R.S.; Ku, B.; Lee, M.T.; et al. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology 2009, 110, 834–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raper, J.; Alvarado, M.C.; Murphy, K.L.; Baxter, M.G. Multiple Anesthetic Exposure in Infant Monkeys Alters Emotional Reactivity to an Acute Stressor. Anesthesiology 2015, 123, 1084–1092. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, M.C.; Murphy, K.L.; Baxter, M.G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br. J. Anaesth. 2017, 119, 517–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flick, R.P.; Katusic, S.K.; Colligan, R.C.; Wilder, R.T.; Voigt, R.G.; Olson, M.D.; Sprung, J.; Weaver, A.L.; Schroeder, D.R.; Warner, D.O. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 2011, 128, e1053–e1061. [Google Scholar] [CrossRef] [Green Version]
- Wilder, R.T.; Flick, R.P.; Sprung, J.; Katusic, S.K.; Barbaresi, W.J.; Mickelson, C.; Gleich, S.J.; Schroeder, D.R.; Weaver, A.L.; Warner, D.O. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 2009, 110, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.S.; Li, G.; Miller, T.L.; Salorio, C.; Byrne, M.W.; Bellinger, D.C.; Ing, C.; Park, R.; Radcliffe, J.; Hays, S.R.; et al. Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood. JAMA 2016, 315, 2312–2320. [Google Scholar] [CrossRef]
- Davidson, A.J.; Disma, N.; de Graaff, J.C.; Withington, D.E.; Dorris, L.; Bell, G.; Stargatt, R.; Bellinger, D.C.; Schuster, T.; Arnup, S.J.; et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet 2016, 387, 239–250. [Google Scholar] [CrossRef] [Green Version]
- Warner, D.S.; McFarlane, C.; Todd, M.M.; Ludwig, P.; McAllister, A.M. Sevoflurane and halothane reduce focal ischemic brain damage in the rat. Possible influence on thermoregulation. Anesthesiology 1993, 79, 985–992. [Google Scholar] [CrossRef]
- Harada, H.; Kelly, P.J.; Cole, D.J.; Drummond, J.C.; Patel, P.M. Isoflurane reduces N-methyl-D-aspartate toxicity in vivo in the rat cerebral cortex. Anesth. Analg. 1999, 89, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Aono, M.; Lee, Y.; Massey, G.; Pearlstein, R.D.; Warner, D.S. Effects of volatile anesthetics on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cultures. Anesthesiology 2001, 95, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Callaway, J.K.; Jones, N.C.; Royse, A.G.; Royse, C.F. Sevoflurane anesthesia does not impair acquisition learning or memory in the Morris water maze in young adult and aged rats. Anesthesiology 2012, 117, 1091–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eizaga Rebollar, R.; Garcia Palacios, M.V.; Morales Guerrero, J.; Torres Morera, L.M. Neurotoxicity versus Neuroprotection of Anesthetics: Young Children on the Ropes? Paediatr. Drugs 2017, 19, 271–275. [Google Scholar] [CrossRef]
- Alam, A.; Suen, K.C.; Hana, Z.; Sanders, R.D.; Maze, M.; Ma, D. Neuroprotection and neurotoxicity in the developing brain: An update on the effects of dexmedetomidine and xenon. Neurotoxicol. Teratol. 2017, 60, 102–116. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhao, H.; Weng, H.; Ma, D. Lasting effects of general anesthetics on the brain in the young and elderly: “mixed picture” of neurotoxicity, neuroprotection and cognitive impairment. J. Anesth. 2019, 33, 321–335. [Google Scholar] [CrossRef] [Green Version]
- Wei, H. The role of calcium dysregulation in anesthetic-mediated neurotoxicity. Anesth. Analg. 2011, 113, 972–974. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 2009, 1793, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef]
- Trychta, K.A.; Back, S.; Henderson, M.J.; Harvey, B.K. KDEL Receptors Are Differentially Regulated to Maintain the ER Proteome under Calcium Deficiency. Cell Rep. 2018, 25, 1829–1840.e6. [Google Scholar] [CrossRef]
- Yang, H.; Liang, G.; Hawkins, B.J.; Madesh, M.; Pierwola, A.; Wei, H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology 2008, 109, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Kang, B.; Wei, W.; Liang, G.; Meng, Q.C.; Li, Y.; Eckenhoff, R.G. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005, 1037, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Wu, X.; Lu, Y.; Xu, Z.; Knapp, A.; Yue, Y.; Xu, T.; Xie, Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J. Biol. Chem. 2010, 285, 4025–4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokubun, H.; Jin, H.; Aoe, T. Pathogenic Effects of Impaired Retrieval between the Endoplasmic Reticulum and Golgi Complex. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; Komita, M.; Aoe, T. The Role of BiP Retrieval by the KDEL Receptor in the Early Secretory Pathway and its Effect on Protein Quality Control and Neurodegeneration. Front. Mol. Neurosci. 2017, 10, 222. [Google Scholar] [CrossRef] [Green Version]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Almanza, A.; Carlesso, A.; Chintha, C.; Creedican, S.; Doultsinos, D.; Leuzzi, B.; Luis, A.; McCarthy, N.; Montibeller, L.; More, S.; et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019, 286, 241–278. [Google Scholar] [CrossRef]
- He, M.; Guo, H.; Yang, X.; Zhou, L.; Zhang, X.; Cheng, L.; Zeng, H.; Hu, F.B.; Tanguay, R.M.; Wu, T. Genetic variations in HSPA8 gene associated with coronary heart disease risk in a Chinese population. PLoS ONE 2010, 5, e9684. [Google Scholar] [CrossRef]
- Minakshi, R.; Rahman, S.; Jan, A.T.; Archana, A.; Kim, J. Implications of aging and the endoplasmic reticulum unfolded protein response on the molecular modality of breast cancer. Exp. Mol. Med. 2017, 49, e389. [Google Scholar] [CrossRef] [Green Version]
- Ogen-Shtern, N.; Ben David, T.; Lederkremer, G.Z. Protein aggregation and ER stress. Brain Res. 2016, 1648, 658–666. [Google Scholar] [CrossRef]
- Kalinderi, K.; Bostantjopoulou, S.; Fidani, L. The genetic background of Parkinson’s disease: Current progress and future prospects. Acta Neurol. Scand. 2016, 134, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Jan, A.T.; Ayyagari, A.; Kim, J.; Kim, J.; Minakshi, R. Entanglement of UPR(ER) in Aging Driven Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.; Archana, A.; Jan, A.T.; Minakshi, R. Dissecting Endoplasmic Reticulum Unfolded Protein Response (UPR(ER)) in Managing Clandestine Modus Operandi of Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komita, M.; Jin, H.; Aoe, T. The effect of endoplasmic reticulum stress on neurotoxicity caused by inhaled anesthetics. Anesth Analg. 2013, 117, 1197–1204. [Google Scholar] [CrossRef]
- Jin, H.; Komita, M.; Aoe, T. Decreased Protein Quality Control Promotes the Cognitive Dysfunction Associated With Aging and Environmental Insults. Front. Neurosci. 2018, 12, 753. [Google Scholar] [CrossRef] [Green Version]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef] [Green Version]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded- protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Lee, K.; Tirasophon, W.; Shen, X.; Michalak, M.; Prywes, R.; Okada, T.; Yoshida, H.; Mori, K.; Kaufman, R.J. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002, 16, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.; Mallucci, G.R. The unfolded protein response in neurodegenerative disorders—Therapeutic modulation of the PERK pathway. FEBS J. 2019, 286, 342–355. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Lewis, M.J.; Pelham, H.R. A human homologue of the yeast HDEL receptor. Nature 1990, 348, 162–163. [Google Scholar] [CrossRef]
- Yamamoto, K.; Fujii, R.; Toyofuku, Y.; Saito, T.; Koseki, H.; Hsu, V.W.; Aoe, T. The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. Embo J. 2001, 20, 3082–3091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mimura, N.; Hamada, H.; Kashio, M.; Jin, H.; Toyama, Y.; Kimura, K.; Iida, M.; Goto, S.; Saisho, H.; Toshimori, K.; et al. Aberrant quality control in the endoplasmic reticulum impairs the biosynthesis of pulmonary surfactant in mice expressing mutant BiP. Cell Death Differ. 2007, 14, 1475–1485. [Google Scholar] [CrossRef]
- Jin, H.; Mimura, N.; Kashio, M.; Koseki, H.; Aoe, T. Late-onset of spinal neurodegeneration in knock-in mice expressing a mutant BiP. PLoS ONE 2014, 9, e112837. [Google Scholar] [CrossRef]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.Y.; Chen, X.Q.; Tang, W.; Wang, Q.X.; Zhang, J. GRP78 silencing enhances hyperoxia-induced alveolar epithelial cell apoptosis via CHOP pathway. Mol. Med. Rep. 2017, 16, 1493–1501. [Google Scholar] [CrossRef]
- Olton, D.S.; Papas, B.C. Spatial memory and hippocampal function. Neuropsychologia 1979, 17, 669–682. [Google Scholar] [CrossRef]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Annachhatre, A.S.; Annachhatre, S.R. Preconditioning in cardiac anesthesia…… where are we? Ann. Card Anaesth. 2019, 22, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Alkhulaifi, A.M.; Pugsley, W.B. Preconditioning the human myocardium. Lancet 1993, 342, 276–277. [Google Scholar] [CrossRef]
- Gidday, J.M.; Fitzgibbons, J.C.; Shah, A.R.; Park, T.S. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci. Lett. 1994, 168, 221–224. [Google Scholar] [CrossRef]
- Lee, H.T.; Lineaweaver, W.C. Protection against ischemic-reperfusion injury of skeletal muscle: Role of ischemic preconditioning and adenosine pretreatment. J. Reconstr. Microsurg. 1996, 12, 383–388. [Google Scholar] [CrossRef]
- Lloris-Carsi, J.M.; Cejalvo, D.; Toledo-Pereyra, L.H.; Calvo, M.A.; Suzuki, S. Preconditioning: Effect upon lesion modulation in warm liver ischemia. Transplant. Proc. 1993, 25, 3303–3304. [Google Scholar]
- Benjamin, I.J.; McMillan, D.R. Stress (heat shock) proteins: Molecular chaperones in cardiovascular biology and disease. Circ. Res. 1998, 83, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Saito, A.; Okuno, S.; Ferrand-Drake, M.; Chan, P.H. Induction of GRP78 by ischemic preconditioning reduces endoplasmic reticulum stress and prevents delayed neuronal cell death. J. Cereb. Blood Flow Metab. 2003, 23, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Orriach, J.L.; Escalona Belmonte, J.J.; Ramirez Fernandez, A.; Ramirez Aliaga, M.; Rubio Navarro, M.; Cruz Manas, J. Cardioprotection with halogenated gases: How does it occur? Drug Des. Dev. Ther. 2017, 11, 837–849. [Google Scholar] [CrossRef] [Green Version]
- Warltier, D.C.; Kersten, J.R.; Pagel, P.S.; Gross, G.J. Anesthetic preconditioning: Serendipity and science. Anesthesiology 2002, 97, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zuo, Z. Isoflurane preconditioning decreases glutamate receptor overactivation-induced Purkinje neuronal injury in rat cerebellar slices. Brain Res. 2005, 1054, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liang, G.; Yang, H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci. Lett. 2007, 425, 59–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Yang, Z.; Liang, G.; Wu, Z.; Peng, Y.; Joseph, D.J.; Inan, S.; Wei, H. Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology 2013, 118, 537–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Deng, B.; Zhao, X.; Gao, C.; Yang, L.; Zhao, H.; Yu, D.; Zhang, F.; Xu, L.; Chen, L.; et al. Isoflurane preconditioning provides neuroprotection against stroke by regulating the expression of the TLR4 signalling pathway to alleviate microglial activation. Sci. Rep. 2015, 5, 11445. [Google Scholar] [CrossRef] [PubMed]
- Bommiasamy, H.; Popko, B. Animal models in the study of the unfolded protein response. Methods Enzymol. 2011, 491, 91–109. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sato, T.; Matsui, T.; Sato, M.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell 2007, 13, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Reimold, A.M.; Etkin, A.; Clauss, I.; Perkins, A.; Friend, D.S.; Zhang, J.; Horton, H.F.; Scott, A.; Orkin, S.H.; Byrne, M.C.; et al. An essential role in liver development for transcription factor XBP-1. Genes Dev. 2000, 14, 152–157. [Google Scholar]
- Zhang, K.; Wong, H.N.; Song, B.; Miller, C.N.; Scheuner, D.; Kaufman, R.J. The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J. Clin. Investig. 2005, 115, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Harding, H.P.; Zeng, H.; Zhang, Y.; Jungries, R.; Chung, P.; Plesken, H.; Sabatini, D.D.; Ron, D. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol. Cell 2001, 7, 1153–1163. [Google Scholar] [CrossRef]
- Gluncic, V.; Moric, M.; Chu, Y.; Hanko, V.; Li, J.; Lukic, I.K.; Lukic, A.; Edassery, S.L.; Kroin, J.S.; Persons, A.L.; et al. In utero Exposure to Anesthetics Alters Neuronal Migration Pattern in Developing Cerebral Cortex and Causes Postnatal Behavioral Deficits in Rats. Cereb. Cortex 2019. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Kubo, K.I.; Nakajima, K. Reelin and Neuropsychiatric Disorders. Front. Cell Neurosci. 2016, 10, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemi, S.H.; Snow, A.V.; Stary, J.M.; Araghi-Niknam, M.; Reutiman, T.J.; Lee, S.; Brooks, A.I.; Pearce, D.A. Reelin signaling is impaired in autism. Biol. Psychiatry 2005, 57, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Mimura, N.; Yuasa, S.; Soma, M.; Jin, H.; Kimura, K.; Goto, S.; Koseki, H.; Aoe, T. Altered quality control in the endoplasmic reticulum causes cortical dysplasia in knock-in mice expressing a mutant BiP. Mol. Cell Biol. 2008, 28, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Dong, Y.; Zhang, J.; Xu, Z.; Wang, G.; Swain, C.A.; Zhang, Y.; Xie, Z. Isoflurane induces endoplasmic reticulum stress and caspase activation through ryanodine receptors. Br. J. Anaesth. 2014, 113, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Xia, J.; Chen, Y.; Zhang, J. Sevoflurane-Induced Endoplasmic Reticulum Stress Contributes to Neuroapoptosis and BACE-1 Expression in the Developing Brain: The Role of eIF2alpha. Neurotox. Res. 2017, 31, 218–229. [Google Scholar] [CrossRef]
- Zhu, G.; Tao, L.; Wang, R.; Xue, Y.; Wang, X.; Yang, S.; Sun, X.; Gao, G.; Mao, Z.; Yang, Q. Endoplasmic reticulum stress mediates distinct impacts of sevoflurane on different subfields of immature hippocampus. J. Neurochem. 2017, 142, 272–285. [Google Scholar] [CrossRef] [Green Version]
- Coghlan, M.; Richards, E.; Shaik, S.; Rossi, P.; Vanama, R.B.; Ahmadi, S.; Petroz, C.; Crawford, M.; Maynes, J.T. Inhalational Anesthetics Induce Neuronal Protein Aggregation and Affect ER Trafficking. Sci. Rep. 2018, 8, 5275. [Google Scholar] [CrossRef]
- Shen, F.Y.; Song, Y.C.; Guo, F.; Xu, Z.D.; Li, Q.; Zhang, B.; Ma, Y.Q.; Zhang, Y.Q.; Lin, R.; Li, Y.; et al. Cognitive Impairment and Endoplasmic Reticulum Stress Induced by Repeated Short-Term Sevoflurane Exposure in Early Life of Rats. Front. Psychiatry 2018, 9, 332. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ou, G.; Chen, Y.; Zhang, J. Inhibition of protein tyrosine phosphatase 1B protects against sevoflurane-induced neurotoxicity mediated by ER stress in developing brain. Brain Res. Bull 2019, 146, 28–39. [Google Scholar] [CrossRef]
- Seo, E.H.; Piao, L.; Park, H.J.; Lee, J.Y.; Sa, M.; Oh, C.S.; Lee, S.H.; Kim, S.H. Impact of general anaesthesia on endoplasmic reticulum stress: Propofol vs. isoflurane. Int. J. Med. Sci. 2019, 16, 1287–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckel, B.; Ohl, F.; Starker, L.; Rammes, G.; Bogdanski, R.; Kochs, E.; Blobner, M. Effects of isoflurane-induced anaesthesia on cognitive performance in a mouse model of Alzheimer’s disease: A randomised trial in transgenic APP23 mice. Eur. J. Anaesthesiol. 2013, 30, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Schoen, J.; Husemann, L.; Tiemeyer, C.; Lueloh, A.; Sedemund-Adib, B.; Berger, K.U.; Hueppe, M.; Heringlake, M. Cognitive function after sevoflurane- vs. propofol-based anaesthesia for on-pump cardiac surgery: A randomized controlled trial. Br. J. Anaesth. 2011, 106, 840–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Shan, G.J.; Zhang, Y.X.; Cao, S.J.; Zhu, S.N.; Li, H.J.; Ma, D.; Wang, D.X.; First Study of Perioperative Organ Protection (SPOP1) investigators. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br. J. Anaesth. 2018, 121, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokkedal, U.; Hansen, T.G.; Rasmussen, L.S.; Mengel-From, J.; Christensen, K. Cognitive Functioning after Surgery in Middle-aged and Elderly Danish Twins. Anesthesiology 2016, 124, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Sprung, J.; Roberts, R.O.; Knopman, D.S.; Price, L.L.; Schulz, H.P.; Tatsuyama, C.L.; Weingarten, T.N.; Schroeder, D.R.; Hanson, A.C.; Petersen, R.C.; et al. Mild Cognitive Impairment and Exposure to General Anesthesia for Surgeries and Procedures: A Population-Based Case-Control Study. Anesth. Analg. 2017, 124, 1277–1290. [Google Scholar] [CrossRef]
- Aiello Bowles, E.J.; Larson, E.B.; Pong, R.P.; Walker, R.L.; Anderson, M.L.; Yu, O.; Gray, S.L.; Crane, P.K.; Dublin, S. Anesthesia Exposure and Risk of Dementia and Alzheimer’s Disease: A Prospective Study. J. Am. Geriatr Soc. 2016, 64, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.W.; Lin, C.C.; Chen, K.B.; Kuo, Y.C.; Li, C.Y.; Chung, C.J. Increased risk of dementia in people with previous exposure to general anesthesia: A nationwide population-based case-control study. Alzheimers Dement. 2014, 10, 196–204. [Google Scholar] [CrossRef]
- Istaphanous, G.K.; Howard, J.; Nan, X.; Hughes, E.A.; McCann, J.C.; McAuliffe, J.J.; Danzer, S.C.; Loepke, A.W. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology 2011, 114, 578–587. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokubun, H.; Jin, H.; Komita, M.; Aoe, T. Conflicting Actions of Inhalational Anesthetics, Neurotoxicity and Neuroprotection, Mediated by the Unfolded Protein Response. Int. J. Mol. Sci. 2020, 21, 450. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020450
Kokubun H, Jin H, Komita M, Aoe T. Conflicting Actions of Inhalational Anesthetics, Neurotoxicity and Neuroprotection, Mediated by the Unfolded Protein Response. International Journal of Molecular Sciences. 2020; 21(2):450. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020450
Chicago/Turabian StyleKokubun, Hiroshi, Hisayo Jin, Mari Komita, and Tomohiko Aoe. 2020. "Conflicting Actions of Inhalational Anesthetics, Neurotoxicity and Neuroprotection, Mediated by the Unfolded Protein Response" International Journal of Molecular Sciences 21, no. 2: 450. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21020450




