Blue Light Mediates Chloroplast Avoidance and Enhances Photoprotection of Vanilla Orchid
Abstract
:1. Introduction
2. Results
2.1. Blue Light Induces Chloroplast Movement in Vanilla Orchid
2.2. Effect of Light and Temperature on Chl F of Vanilla Orchid
2.3. Extended BL-Acclimation Duration Enhances Chl F Capacity
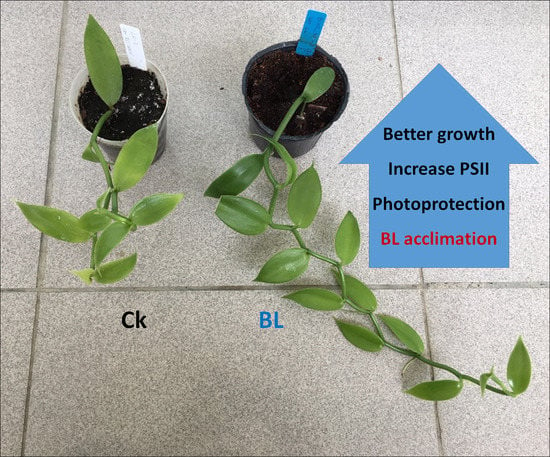
2.4. Effect of Blue Light Acclimation on the Growth of Vanilla Orchid
2.4.1. BL-Acclimation Enhances Growth and Induces Chloroplast Avoidance
2.4.2. BL-Acclimation Increases Antioxidant Capacity and Sugar Content
2.4.3. BL-Acclimation Vanilla Orchid Altered Gene Expression Patterns
2.5. BL-Acclimation Increases Photoprotection of Vanilla Orchid
2.5.1. BL-Acclimation Enhances Growth and Increases Chl F Capacity
2.5.2. Effect of BL-Acclimation on Antioxidative Enzymes and Sugar Content under High Irradiation
2.5.3. BL-Acclimation Alters Gene Expression Patterns under High Irradiation
3. Discussion
3.1. Blue Light Mediates Chloroplast Avoidance in Vanilla Orchid
3.2. Vanilla Orchid is Tolerant to Moderately High Temperature but Sensitive to High Irradiation
3.3. Impact of Blue Light Acclimation on Vanilla Growth
3.3.1. BL-Acclimation Causes Chloroplast Avoidance to Reduce Photoinhibition
3.3.2. BL-Acclimation Reduces ROS and Enhances Antioxidants
3.3.3. BL-Acclimation Increases Photosynthesis-Related Genes under High Light Stress
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Chloroplast Movement Using SPAD Measurement
4.3. Measurement of Chlorophyll Fluorescence Parameters
4.4. Measurement of Antioxidant Enzyme Activities and Sugar Content
4.5. Gene Expression Analysis
4.6. Statistical Analyses
5. Conclusion and Perspective
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BL | Blue light |
| ML | Moderate light |
| HL | High light |
| PSI | Photosystem I |
| PSII | Photosystem II |
| Fv/Fm | Maximal quantum efficiency of PSⅡ photochemistry |
| ETR | Electron transport rate |
| NPQ | Nonphotochemical quenching |
| qP | Photochemical quenching of fluorescence |
| qN | Nonphotochemical quenching coefficient |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| ROS | Reactive oxygen species |
References
- Fouche, J.G.; Jouve, L. Vanilla planifolia: History, botany and culture in Reunion island. Agronomie 1999, 19, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Bory, S.; Grisoni, M.; Duval, M.-F.; Besse, P. Biodiversity and preservation of vanilla: Present state of knowledge. Genet. Resour. Crop Evol. 2007, 55, 551–571. [Google Scholar] [CrossRef]
- Barrow, S.R.; Cockburn, W. Effects of light quantity and quality on the decarboxylation of malic Acid in crassulacean Acid metabolism photosynthesis. Plant Physiol. 1982, 69, 568–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havkin-Frenkel, D.; Belanger, F.C. Handbook of Vanilla Science and Technology, 2nd ed.; Wiley-Blackwell: Chichester, UK, 2019. [Google Scholar]
- Díez, M.C.; Moreno, F.; Gantiva, E. Effects of light intensity on the morphology and CAM photosynthesis of Vanilla planifolia Andrews. Rev. Fac. Nac. Agron. 2017, 70, 8023–8033. [Google Scholar] [CrossRef]
- Johnson, M.P. Photosynthesis. Essays Biochem. 2016, 60, 255–273. [Google Scholar] [CrossRef]
- Scheller, H.V.; Haldrup, A. Photoinhibition of photosystem I. Planta 2005, 221, 5–8. [Google Scholar] [CrossRef]
- Bhattacharjee, S. ROS and Regulation of Photosynthesis. In Reactive Oxygen Species in Plant Biology; Springer India: New Delhi, India, 2019; pp. 107–125. [Google Scholar] [CrossRef]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]
- Kong, S.G.; Wada, M. Molecular basis of chloroplast photorelocation movement. J. Plant Res. 2016, 129, 159–166. [Google Scholar] [CrossRef]
- Krzeszowiec, W.; Novokreshchenova, M.; Gabryś, H. Chloroplasts in C3 grasses move in response to blue-light. Plant Cell Rep. 2020. [Google Scholar] [CrossRef]
- Lin, Y.J.; Chen, Y.C.; Tseng, K.C.; Chang, W.C.; Ko, S.S. Phototropins mediate chloroplast movement in Phalaenopsis aphrodite (moth orchid). Plant Cell Physiol. 2019, 60, 2243–2254. [Google Scholar] [CrossRef]
- Murchie, E.H.; Niyogi, K.K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Aro, E.M.; Millar, A.H. Mechanisms of Photodamage and Protein Turnover in Photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chen, S.T.; He, N.Y.; Wang, Q.L.; Zhao, Y.; Gao, W.; Guo, F.Q. Nuclear-encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat. Plants 2020. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; McAusland, L.; Lawson, T.; Raines, C.A. Overexpression of the RieskeFeS Protein Increases Electron Transport Rates and Biomass Yield. Plant Physiol. 2017, 175, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, M.; Lopez-Calcagno, P.E.; Raines, C.A.; Furbank, R.T.; von Caemmerer, S. Overexpression of the Rieske FeS protein of the Cytochrome b 6 f complex increases C4 photosynthesis in Setaria viridis. Commun. Biol. 2019, 2, 314. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J. Genetic Engineering for Global Food Security: Photosynthesis and Biofortification. Plants 2019, 8, 586. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Dong, Y.; Dijkwel, P.P.; Mueller-Roeber, B.; Gechev, T.S. Genome-Wide Analysis of ROS Antioxidant Genes in Resurrection Species Suggest an Involvement of Distinct ROS Detoxification Systems during Desiccation. Int. J. Mol. Sci. 2019, 20, 3101. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.-S.; Jhong, C.-M.; Shih, M.-C. Blue Light Acclimation Reduces the Photoinhibition of Phalaenopsis aphrodite (Moth Orchid). Int. J. Mol. Sci. 2020, 21, 6167. [Google Scholar] [CrossRef]
- Jarillo, J.A.; Gabrys, H.; Capel, J.; Alonso, J.M.; Ecker, J.R.; Cashmore, A.R. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 2001, 410, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Sakai, T.; Suetsugu, N.; Oikawa, K.; Ishiguro, S.; Kato, T.; Tabata, S.; Okada, K.; Wada, M. Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 2001, 291, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.G.; Arai, Y.; Suetsugu, N.; Yanagida, T.; Wada, M. Rapid severing and motility of chloroplast-actin filaments are required for the chloroplast avoidance response in Arabidopsis. Plant Cell 2013, 25, 572–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burritt, D.J.; Mackenzie, S. Antioxidant metabolism during acclimation of Begonia × erythrophylla to high light levels. Ann. Bot. 2003, 91, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Kurata, K. Effects of blue light deficiency on acclimation of light energy partitioning in PSII and CO2 assimilation capacity to high irradiance in spinach leaves. Plant Cell Physiol. 2008, 49, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Walters, R.G.; Horton, P. Acclimation of Arabidopsis thaliana to the light environment: Regulation of chloroplast composition. Planta 1995, 197, 475–481. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; Noga, G.; Hunsche, M. High blue light improves acclimation and photosynthetic recovery of pepper plants exposed to UV stress. Environ. Exp. Bot. 2015, 109, 254–263. [Google Scholar] [CrossRef]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast avoidance movement reduces photodamage in plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef]
- Steyn, W.J. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Wen, F.; Xing, D.; Zhang, L. Hydrogen peroxide is involved in high blue light-induced chloroplast avoidance movements in Arabidopsis. J. Exp. Bot. 2008, 59, 2891–2901. [Google Scholar] [CrossRef] [Green Version]
- Rossatto, T.; do Amaral, M.N.; Benitez, L.C.; Vighi, I.L.; Braga, E.J.B.; de Magalhaes Junior, A.M.; Maia, M.A.C.; da Silva Pinto, L. Gene expression and activity of antioxidant enzymes in rice plants, cv. BRS AG, under saline stress. Physiol. Mol. Biol. Plants 2017, 23, 865–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamse, P. Amelioration of Uv-B Damage Under High Irradiance. II: Role of Blue Light Photoreceptors. Photochem. Photobiol. 1994, 2, 110–115. [Google Scholar] [CrossRef]
- Zhang, N.; Kallis, R.P.; Ewy, R.G.; Portis, A.R., Jr. Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc. Natl. Acad. Sci. USA 2002, 99, 3330–3334. [Google Scholar] [CrossRef] [Green Version]
- Cardona, T.; Shao, S.; Nixon, P.J. Enhancing photosynthesis in plants: The light reactions. Essays Biochem. 2018, 62, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef]








| Source | df | L3 | LM |
|---|---|---|---|
| Model | 29 | 0.0665 *** | 0.0204 *** |
| BL | 5 | 0.3737 *** | 0.1057 *** |
| Time | 4 | 0.0012 ns | 0.0062 ns |
| BL*Time | 20 | 0.0027 ns | 0.0019 ns |
| Error | 120 | 0.0035 | 0.0027 |
| Total | 149 |
| BL Day | Light | Fv/Fm | ETR | NPQ | qP | qN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 day | 150 | 0.74 | a | 9.50 | a | 2.05 | b | 0.32 | a | 0.81 | b | |
| 500 | 0.75 | a | 7.08 | ab | 2.59 | ab | 0.08 | b | 0.85 | ab | ||
| 1000 | 0.74 | a | 5.35 | b | 2.82 | a | 0.03 | b | 0.88 | a | ||
| 4 day | 150 | 0.74 | a | 15.75 | a | 2.50 | a | 0.60 | a | 0.86 | b | |
| 500 | 0.73 | a | 16.97 | a | 3.06 | a | 0.22 | b | 0.90 | a | ||
| 1000 | 0.72 | a | 7.82 | b | 3.03 | a | 0.06 | c | 0.90 | a | ||
| 8 day | 150 | 0.70 | a | 19.62 | a | 2.13 | b | 0.65 | a | 0.81 | b | |
| 500 | 0.70 | a | 16.63 | a | 3.11 | a | 0.24 | b | 0.90 | a | ||
| 1000 | 0.72 | a | 16.25 | a | 3.36 | a | 0.13 | c | 0.91 | a | ||
| 12 day | 150 | 0.71 | a | 17.02 | a | 2.53 | b | 0.64 | a | 0.85 | b | |
| 500 | 0.71 | a | 16.08 | a | 3.14 | ab | 0.23 | b | 0.90 | a | ||
| 1000 | 0.68 | a | 10.32 | b | 3.30 | a | 0.08 | c | 0.92 | a | ||
| df | ||||||||||||
| BLday | 3 | ** | *** | * | *** | *** | ||||||
| Light | 2 | n.s. | *** | *** | *** | *** | ||||||
| BLday × Light | 6 | n.s. | * | n.s. | *** | n.s. | ||||||
| Light | BL_Ac | df | F/vFm | ETR | NPQ | qP | qN | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ML500 | BL | 0.59 | a | 7.03 | a | 2.03 | a | 0.36 | a | 0.87 | a | |
| CK | 0.61 | a | 7.99 | a | 2.12 | a | 0.34 | a | 0.85 | a | ||
| HL1000 | BL | 0.42 | a | 5.65 | a | 1.98 | a | 0.35 | a | 0.89 | a | |
| CK | 0.08 | b | −0.56 | b | 0.58 | b | −0.34 | a | 0.81 | a | ||
| Light | 1 | *** | * | * | * | n.s. | ||||||
| BL_Ac | 1 | * | n.s. | n.s. | * | n.s. | ||||||
| Light × BL_Ac | 1 | * | n.s. | * | * | n.s. | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, S.-S.; Jhong, C.-M.; Lin, Y.-J.; Wei, C.-Y.; Lee, J.-Y.; Shih, M.-C. Blue Light Mediates Chloroplast Avoidance and Enhances Photoprotection of Vanilla Orchid. Int. J. Mol. Sci. 2020, 21, 8022. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218022
Ko S-S, Jhong C-M, Lin Y-J, Wei C-Y, Lee J-Y, Shih M-C. Blue Light Mediates Chloroplast Avoidance and Enhances Photoprotection of Vanilla Orchid. International Journal of Molecular Sciences. 2020; 21(21):8022. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218022
Chicago/Turabian StyleKo, Swee-Suak, Chung-Min Jhong, Yi-Jyun Lin, Ching-Yu Wei, Ju-Yin Lee, and Ming-Che Shih. 2020. "Blue Light Mediates Chloroplast Avoidance and Enhances Photoprotection of Vanilla Orchid" International Journal of Molecular Sciences 21, no. 21: 8022. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218022