Aβ Beyond the AD Pathology: Exploring the Structural Response of Membranes Exposed to Nascent Aβ Peptide
Abstract
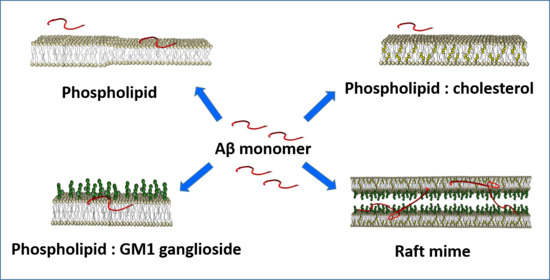
:1. Introduction
2. Results and Discussion
2.1. Systems and Experimental Conditions
2.2. Single-Component PC-Phospholipid Bilayers
2.3. Multicomponent PC-Phospholipid-Based Bilayers
2.3.1. Mixed PC-Phospholipid /GM1-Ganglioside Bilayers
2.3.2. Mixed PC-Phospholipid/Cholesterol Bilayers
2.3.3. Mixed PC-Phospholipid/Cholesterol/GM1 (Raft-Mime) Bilayers: From Intra-Membrane Restructuring to Inter-Membrane Correlation
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morley, J.E.; Farr, S.A.; Nguyen, A.D.; Xu, F. What is the Physiological Function of Amyloid-Beta Protein? J. Nutr. Health Aging 2019, 23, 225–226. [Google Scholar] [CrossRef] [Green Version]
- Puzzo, D.; Gulisano, W.; Arancio, O.; Palmeri, A. The keystone of Alzheimer pathogenesis might be sought in Aβ physiology. Neuroscience 2015, 307, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Gosztyla, M.L.; Brothers, H.M.; Robinson, S.R. Alzheimer’s Amyloid-β is an Antimicrobial Peptide: A Review of the Evidence. J. Alzheimers Dis. 2018, 62, 1495–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuffrida, M.L.; Caraci, F.; Pignataro, B.; Cataldo, S.; De Bona, P.; Bruno, V.; Molinaro, G.; Pappalardo, G.; Messina, A.; Palmigiano, A.; et al. Beta-Amyloid Monomers Are Neuroprotective. J. Neurosci. 2009, 29, 10582–10587. [Google Scholar] [CrossRef]
- Copani, A. The underexplored question of β-amyloid monomers. Eur. J. Pharmacol. 2017, 817, 71–75. [Google Scholar] [CrossRef]
- Greenwald, J.; Riek, R. On the Possible Amyloid Origin of Protein Folds. J. Mol. Biol. 2012, 421, 417–426. [Google Scholar] [CrossRef]
- Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterfield, S.M.; Lashuel, H.A. Amyloidogenic Protein-Membrane Interactions: Mechanistic Insight from Model Systems. Angew. Chem. Int. Ed. 2010, 49, 5628–5654. [Google Scholar] [CrossRef]
- Gilson, V.; Mbebi-Liegeois, C.; Sellal, F.; de Barry, J. Effects of Low Amyloid-β (Aβ) Concentration on Aβ1–42 Oligomers Binding and GluN2B Membrane Expression. J. Alzheimers Dis. 2015, 47, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.C.; Nam, E.; Lee, H.J.; Savelieff, M.G.; Lim, M.H. Towards an understanding of amyloid-β oligomers: Characterization, toxicity mechanisms, and inhibitors. Chem. Soc. Rev. 2017, 46, 310–323. [Google Scholar] [CrossRef]
- Williams, T.L.; Serpell, L.C. Membrane and surface interactions of Alzheimer’s Aβ peptide-insights into the mechanism of cytotoxicity: Membrane interactions of Alzheimer’s Aβ peptide. FEBS J. 2011, 278, 3905–3917. [Google Scholar] [CrossRef] [PubMed]
- Serra-Batiste, M.; Ninot-Pedrosa, M.; Bayoumi, M.; Gairí, M.; Maglia, G.; Carulla, N. Aβ42 assembles into specific β-barrel pore-forming oligomers in membrane-mimicking environments. Proc. Natl. Acad. Sci. USA 2016, 113, 10866–10871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindberg, D.J.; Wesén, E.; Björkeroth, J.; Rocha, S.; Esbjörner, E.K. Lipid membranes catalyse the fibril formation of the amyloid-β (1–42) peptide through lipid-fibril interactions that reinforce secondary pathways. Biochim. Biophys. Acta BBA-Biomembr. 2017, 1859, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Kotler, S.A.; Walsh, P.; Brender, J.R.; Ramamoorthy, A. Differences between amyloid-β aggregation in solution and on the membrane: Insights into elucidation of the mechanistic details of Alzheimer’s disease. Chem. Soc. Rev. 2014, 43, 6692–6700. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K.; Kato, K.; Yanagisawa, K. Aβ polymerization through interaction with membrane gangliosides. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2010, 1801, 868–877. [Google Scholar] [CrossRef]
- Ikeda, K.; Yamaguchi, T.; Fukunaga, S.; Hoshino, M.; Matsuzaki, K. Mechanism of Amyloid β-Protein Aggregation Mediated by GM1 Ganglioside Clusters. Biochemistry (Mosc.) 2011, 50, 6433–6440. [Google Scholar] [CrossRef]
- Stravalaci, M.; Bastone, A.; Beeg, M.; Cagnotto, A.; Colombo, L.; Di Fede, G.; Tagliavini, F.; Cantù, L.; Del Favero, E.; Mazzanti, M.; et al. Specific Recognition of Biologically Active Amyloid-β Oligomers by a New Surface Plasmon Resonance-based Immunoassay and an in Vivo Assay in Caenorhabditis elegans. J. Biol. Chem. 2012, 287, 27796–27805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Shokri-Kojori, E.; Wang, G.-J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brothers, H.M.; Gosztyla, M.L.; Robinson, S.R. The Physiological Roles of Amyloid-β Peptide Hint at New Ways to Treat Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 118. [Google Scholar] [CrossRef]
- Vahed, M.; Neya, S.; Matsuzaki, K.; Hoshino, T. Analysis of Physicochemical Interaction of Aβ40 with a GM1 Ganglioside-Containing Lipid Membrane. J. Phys. Chem. B 2018, 122, 3771–3781. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Sohma, Y.; Hirayama, Y.; Mukai, H.; Kimura, T.; Hayashi, Y.; Matsuzaki, K.; Kiso, Y. “Click Peptide”: pH-Triggered in Situ Production and Aggregation of Monomer Aβ1-42. ChemBioChem 2009, 10, 710–715. [Google Scholar] [CrossRef]
- Pike, L.J. Rafts defined: A report on the Keystone symposium on lipid rafts and cell function. J. Lipid Res. 2006, 47, 1597–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantù, L.; Del Favero, E.; Sonnino, S.; Prinetti, A. Gangliosides and the multiscale modulation of membrane structure. Chem. Phys. Lipids 2011, 164, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Witkowski, J.M.; Bourgade, K.; Khalil, A.; Zerif, E.; Larbi, A.; Hirokawa, K.; Pawelec, G.; Bocti, C.; Lacombe, G.; et al. Can an Infection Hypothesis Explain the Beta Amyloid Hypothesis of Alzheimer’s Disease? Front. Aging Neurosci. 2018, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta BBA-Rev. Biomembr. 2000, 1469, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Brocca, P.; Cantù, L.; Corti, M.; Del Favero, E.; Motta, S.; Nodari, M.C. Curved single-bilayers in the region of the anomalous swelling: Effect of curvature and chain length. Colloids Surf. Physicochem. Eng. Asp. 2006, 291, 63–68. [Google Scholar] [CrossRef]
- Ionov, M.; Klajnert, B.; Gardikis, K.; Hatziantoniou, S.; Palecz, B.; Salakhutdinov, B.; Cladera, J.; Zamaraeva, M.; Demetzos, C.; Bryszewska, M. Effect of amyloid beta peptides Aβ1–28 and Aβ25–40 on model lipid membranes. J. Therm. Anal. Calorim. 2010, 99, 741–747. [Google Scholar] [CrossRef]
- Rondelli, V.; Brocca, P.; Motta, S.; Messa, M.; Colombo, L.; Salmona, M.; Fragneto, G.; Cantù, L.; Del Favero, E. Amyloidβ Peptides in interaction with raft-mime model membranes: A neutron reflectivity insight. Sci. Rep. 2016, 6, 20997. [Google Scholar] [CrossRef] [Green Version]
- Fantini, J.; Yahi, N. Molecular insights into amyloid regulation by membrane cholesterol and sphingolipids: Common mechanisms in neurodegenerative diseases. Expert Rev. Mol. Med. 2010, 12, e27. [Google Scholar] [CrossRef]
- Castorph, S.; Arleth, L.; Sztucki, M.; Vainio, U.; Ghosh, S.K.; Holt, M.; Jahn, R.; Salditt, T. Synaptic Vesicles Studied by SAXS: Derivation and Validation of a Model Form Factor. J. Phys. Conf. Ser. 2010, 247, 012015. [Google Scholar] [CrossRef]
- Vacklin, H.P.; Tiberg, F.; Fragneto, G.; Thomas, R.K. Phospholipase A 2 Hydrolysis of Supported Phospholipid Bilayers: A Neutron Reflectivity and Ellipsometry Study. Biochemistry (Mosc.) 2005, 44, 2811–2821. [Google Scholar] [CrossRef]
- Mouritsen, O.G. The liquid-ordered state comes of age. Biochim. Biophys. Acta BBA-Biomembr. 2010, 1798, 1286–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, W.G.; Li, L.; Müller, W.E.; Eckert, G.P. Cholesterol as a causative factor in Alzheimer’s disease: A debatable hypothesis. J. Neurochem. 2014, 129, 559–572. [Google Scholar] [CrossRef] [Green Version]
- Barrett, P.J.; Song, Y.; Van Horn, W.D.; Hustedt, E.J.; Schafer, J.M.; Hadziselimovic, A.; Beel, A.J.; Sanders, C.R. The Amyloid Precursor Protein Has a Flexible Transmembrane Domain and Binds Cholesterol. Science 2012, 336, 1168–1171. [Google Scholar] [CrossRef] [Green Version]
- Pannuzzo, M. On the physiological/pathological link between Aβ peptide, cholesterol, calcium ions and membrane deformation: A molecular dynamics study. Biochim. Biophys. Acta BBA-Biomembr. 2016, 1858, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Rondelli, V.; Fragneto, G.; Motta, S.; Del Favero, E.; Brocca, P.; Sonnino, S.; Cantù, L. Ganglioside GM1 forces the redistribution of cholesterol in a biomimetic membrane. Biochim. Biophys. Acta BBA-Biomembr. 2012, 1818, 2860–2867. [Google Scholar] [CrossRef] [Green Version]
- Fantini, J.; Yahi, N.; Garmy, N. Cholesterol accelerates the binding of Alzheimer’s β-amyloid peptide to ganglioside GM1 through a universal hydrogen-bond-dependent sterol tuning of glycolipid conformation. Front. Physiol. 2013, 4, 120. [Google Scholar] [CrossRef] [Green Version]
- Regina Todeschini, A.; Hakomori, S. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta BBA-Gen. Subj. 2008, 1780, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Del Favero, E.; Raudino, A.; Brocca, P.; Motta, S.; Fragneto, G.; Corti, M.; Cantú, L. Lamellar Stacking Split by In-Membrane Clustering of Bulky Glycolipids. Langmuir 2009, 25, 4190–4197. [Google Scholar] [CrossRef]
- Mui, B.L.; Cullis, P.R.; Evans, E.A.; Madden, T.D. Osmotic properties of large unilamellar vesicles prepared by extrusion. Biophys. J. 1993, 64, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Tettamanti, G.; Bonali, F.; Marchesini, S.; Zambotti, V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim. Biophys. Acta BBA-Lipids Lipid Metab. 1973, 296, 160–170. [Google Scholar] [CrossRef]
- Daillant, J.; Bellet-Amalric, E.; Braslau, A.; Charitat, T.; Fragneto, G.; Graner, F.; Mora, S.; Rieutord, F.; Stidder, B. Structure and fluctuations of a single floating lipid bilayer. Proc. Natl. Acad. Sci. USA 2005, 102, 11639–11644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brûlet, A.; Lairez, D.; Lapp, A.; Cotton, J.-P. Improvement of data treatment in small-angle neutron scattering. J. Appl. Crystallogr. 2007, 40, 165–177. [Google Scholar] [CrossRef]
- Doucet, M.; Cho, J.H.; Alina, G.; Bakker, J.; Bouwman, W.; Butler, P.; Campbell, K.; Gonzales, M.; Heenan, R.; Jackson, A.; et al. SasView Version 4.2.1; Zenodo: Geneva, Switzerland, 2018. [Google Scholar] [CrossRef]
- Salvetti, G.; Cardelli, C.; Ferrari, C.; Tombari, E. A modulated adiabatic scanning calorimeter (MASC). Thermochim. Acta 2000, 364, 11–22. [Google Scholar] [CrossRef]
- Nelson, A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J. Appl. Crystallogr. 2006, 39, 273–276. [Google Scholar] [CrossRef]
- Taraboletti, G.; Perin, L.; Bottazzi, B.; Mantovani, A.; Giavazzi, R.; Salmona, M. Membrane fluidity affects tumor-cell motility, invasion and lung-colonizing potential. Int. J. Cancer 1989, 44, 707–713. [Google Scholar] [CrossRef]
- Wood, P.L. Lipidomics of Alzheimer’s disease: Current status. Alzheimers Res. Ther. 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso, M.; Bonetto, V. Extracellular Vesicles and a Novel Form of Communication in the Brain. Front. Neurosci. 2016, 10, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Sun, G.Y.; Eckert, G.P.; Lee, J.C.-M. Cellular Membrane Fluidity in Amyloid Precursor Protein Processing. Mol. Neurobiol. 2014, 50, 119–129. [Google Scholar] [CrossRef]
- Svennerholm, L.; Boström, K.; Jungbjer, B. Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathol. (Berl.) 1997, 94, 345–352. [Google Scholar] [CrossRef]
- Svennerholm, L.; Boström, K.; Jungbjer, B.; Olsson, L. Membrane Lipids of Adult Human Brain: Lipid Composition of Frontal and Temporal Lobe in Subjects of Age 20 to 100 Years. J. Neurochem. 2002, 63, 1802–1811. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondelli, V.; Salmona, M.; Colombo, L.; Fragneto, G.; Fadda, G.C.; Cantu’, L.; Del Favero, E. Aβ Beyond the AD Pathology: Exploring the Structural Response of Membranes Exposed to Nascent Aβ Peptide. Int. J. Mol. Sci. 2020, 21, 8295. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218295
Rondelli V, Salmona M, Colombo L, Fragneto G, Fadda GC, Cantu’ L, Del Favero E. Aβ Beyond the AD Pathology: Exploring the Structural Response of Membranes Exposed to Nascent Aβ Peptide. International Journal of Molecular Sciences. 2020; 21(21):8295. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218295
Chicago/Turabian StyleRondelli, Valeria, Mario Salmona, Laura Colombo, Giovanna Fragneto, Giulia C. Fadda, Laura Cantu’, and Elena Del Favero. 2020. "Aβ Beyond the AD Pathology: Exploring the Structural Response of Membranes Exposed to Nascent Aβ Peptide" International Journal of Molecular Sciences 21, no. 21: 8295. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21218295