Eleutherine bulbosa (Mill.) Urb. Bulb: Review of the Pharmacological Activities and Its Prospects for Application
Abstract
:1. Introduction
2. Morphological Characteristics
3. Traditional Uses
4. Phytochemical Constituents
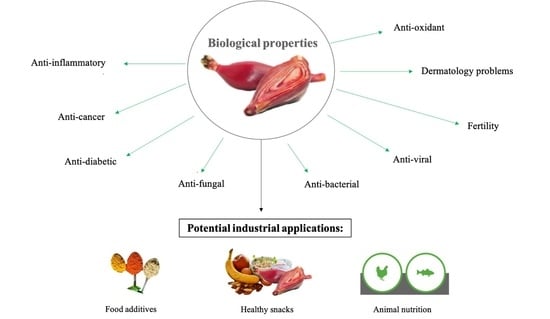
5. Pharmacological Activities
5.1. Chemotherapeutics: Potential Cytotoxic Agent
5.2. Endocrine Disorder: Diabetes Mellitus
5.2.1. In Vitro Studies
5.2.2. In Vivo Studies
5.3. Infectious Disease: Microbial Activity
5.3.1. Anti-Bacterial Properties
5.3.2. Anti-Fungal Properties
5.3.3. Anti-Viral Properties
5.3.4. Anti-Malarial Properties
5.4. Anti-Inflammatory Activities
5.4.1. Rheumatoid Arthritis
5.4.2. Erythrocyte Membrane Stabilization
5.4.3. Lipopolysaccharide-Induced (LPO) Nitric Oxide Production
5.4.4. Activation of CD4+ T Helper (Th) Cells
5.5. Dermatological Conditions
5.5.1. Wound Healing Properties
5.5.2. Anti-Melanogenesis Activity
5.6. Antioxidant Activity
5.7. Effect on the Reproductive System Disorders
6. The Potential Use of E. bulbosa Bulb in Various Applications
6.1. Food Industry
6.1.1. Food Additives
6.1.2. Healthy Snacks
6.2. Feed Additives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusuma, I.W.; Arung, E.T.; Rosamah, E.; Purwatiningsih, S.; Kuspradini, H.; Astuti, J.; Kim, Y.U.; Shimizu, K. Antidermatophyte and antimelanogenesis compound from Eleutherine americana grown in Indonesia. J. Nat. Med. 2010, 64, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Insanu, M.; Kusmardiyani, S.; Hartati, R. Recent studies on phytochemicals and pharmacological effects of Eleutherine americana Merr. Procedia Chem. 2014, 13, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Ieyama, T.; Gunawan-Puteri, M.D.; Kawabata, J. α-Glucosidase inhibitors from the bulb of Eleutherine americana. Food Chem. 2011, 128, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.L.; Moraes, D.F.; Cartagenes, M.; Do Amaral, F.M.; Guerra, R.N. Eleutherine bulbous (Mill.) Urb.: A review study. J. Med. Plants Res. 2016, 10, 286–297. [Google Scholar]
- Rivella, J. Plantas da Amazonia: Oportunidades Economicas e Sustentaveis. Manaus: SEBRAE-AM/INPA 2000. 405p. Available online: https://www.teses.usp.br/teses/disponiveis/8/8136/tde-13052008-154603/publico/MESTRADO_LAIS_MOURAO_MIGUEL.pdf (accessed on 10 June 2021).
- Goldblatt, P.; Le Thomas, A. Pollen apertures, exine sculpturing and phylogeny in Iridaceae subfamily Iridoideae. Rev. Palaeobot. Palyno. 1992, 75, 301–315. [Google Scholar] [CrossRef]
- Kuntorini, E.M.; Nugroho, L.H. Structural development and bioactive content of red bulb plant (Eleutherine americana); a traditional medicines for local Kalimantan people. Biodiversitas J. Bio. Div. 2010, 11, 102–106. [Google Scholar]
- Ifesan, B.O.T.; Joycharat, N.; Voravuthikunchai, S.P. The mode of antistaphylococcal action of Eleutherine americana. FEMS Immunol. Med. Microbiol. 2009, 57, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Zhengxiong, C.; Huizhu, H.; Chengrui, W.; Yuhui, L.I.; Jianmi, D.; Sankawa, U.; Noguchi, H.; Iitaka, Y. Hongconin, a new naphthalene derivative from Hong-Cong, the rhizome of Eleutherine ameicana Merr. et Heyne (Iridaceae). Chem. Pharm. Bull. 1986, 34, 2743–2746. [Google Scholar] [CrossRef] [Green Version]
- Weniger, B.; Haag-Berrurier, M.; Anton, R. Plants of Haiti used as antifertility agents. J. Ethnopharmacol. 1982, 6, 67–84. [Google Scholar] [CrossRef]
- Lans, C. Comparison of plants used for skin and stomach problems in Trinidad and Tobago with Asian ethnomedicine. J. Ethnobiol. Ethnomed. 2007, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lucena, G.M.D.S.; Franco, J.L.; Ribas, C.M.; Azevedo, M.S.; Meotti, F.C.; Gadotti, V.M.; Dafre, A.L.; Santos, A.R.S.; Farina, M. Cipura paludosa extract prevents methyl mercury-induced neurotoxicity in mice. Basic Clin. Pharmacol. Toxicol. 2007, 101, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Tessele, P.B.; Delle Monache, F.; Quintão, N.L.; da Silva, G.F.; Rocha, L.W.; Lucena, G.M.; Ferreira, V.M.; Prediger, R.D.; Cechinel Filho, V. A new naphthoquinone isolated from the bulbs of Cipura paludosa and pharmacological activity of two main constituents. Planta Med. 2011, 77, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.F.; Fernández, I.D.; Maldonado, H.; Torres, R.; Zavaleta, A.; Vaisberg, A.J.; Hammond, G.B. Evaluation of the wound-healing activity of selected traditional medicinal plants from Peru. J. Ethnopharmacol. 1997, 55, 193–200. [Google Scholar] [CrossRef]
- World Health Organization. Medicinal Plants in Viet Nam; WHO Regional Office for the Western Pacific: Manila, Philippines, 1990. [Google Scholar]
- Hara, H.; Maruyama, N.; Yamashita, S.; Hayashi, Y.; Lee, K.H.; Bastow, K.F.; Marumoto, C.R.; Imakura, Y. Elecanacin, a novel new naphthoquinone from the bulb of Eleutherine americana. Chem. Pharm. Bull. 1997, 45, 1714–1716. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, C.; Ceriotti, G. Chemical and pharmacological investigations of constituents of Eleutherine bulbosa (Miller) Urb. (Iridaceae). J. Pharm. Sci. 1975, 64, 1305–1308. [Google Scholar] [CrossRef]
- Gallo, F.R.; Palazzino, G.; Federici, E.; Iurilli, R.; Galeffi, C.; Chifundera, K.; Nicoletti, M. Polyketides from Eleutherine bulbosa. Nat. Prod. Res. 2010, 24, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qiu, F.; Duan, W.; Qu, G.; Wang, N.; Yao, X. New bioactive constituents from Eleutherine americana. Front. Chem. China 2006, 1, 320–323. [Google Scholar] [CrossRef]
- Kamarudin, A.A.; Esa, N.M.; Saad, N.; Sayuti, N.H.; Razak, N.A.A. Heat assisted extraction of phenolic compounds from Eleutherine bulbosa (Mill.) bulb and its bioactive profiles using response surface methodology. Ind. Crop Prod. 2020, 144, 112064. [Google Scholar] [CrossRef]
- Chen, D.L.; Hu, M.G.; Liu, Y.Y.; Li, R.T.; Yu, M.; Xu, X.D.; Ma, G.X. New naphthalene derivatives from the bulbs of Eleutherine americana with their protective effect on the injury of HUVECs. Molecules 2018, 23, 2111. [Google Scholar] [CrossRef] [Green Version]
- Paramapojn, S.; Ganzera, M.; Gritsanapan, W.; Stuppner, H. Analysis of naphthoquinone derivatives in the Asian medicinal plant Eleutherine americana by RP-HPLC and LC–MS. J. Pharm. Biomed. Anal. 2008, 47, 990–993. [Google Scholar] [CrossRef]
- Alves, T.M.A.; Kloos, H.; Zani, C.L. Eleutherinone, a novel fungitoxic naphthoquinone from Eleutherine bulbosa (Iridaceae). Memórias do Inst. Oswaldo Cruz 2003, 98, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Sun, Z.; Liu, Y.; Li, Z.; Liang, H.; Chen, L.; Xu, X.; Yang, J.; Ma, G.; Huo, X. Eleucanainones A and B: Two dimeric structures from the bulbs of Eleutherine americana with anti-MRSA activity. Org. Lett. 2020, 22, 3449–3453. [Google Scholar] [CrossRef]
- Lestari, D.; Kartika, R.; Marliana, E. Antioxidant and anticancer activity of Eleutherine bulbosa (Mill.) Urb on leukemia cells L1210. J. Phys. Conf. Ser. 2019, 1277, 012022. [Google Scholar] [CrossRef] [Green Version]
- Mutiah, R.; Choiroh, F.; Annisa, R.; Listiyana, A. Combinational effect of Eleutherine palmifolia (L.) Merr extract and doxorubicin chemotherapy on HeLa cervical cancer cells. In AIP Conference Proceedings July 2019; AIP Publishing LLC.: New York, NY, USA, 20 July 2019; Volume 2120, No. 1; p. 070001. [Google Scholar]
- Mutiah, R.; Listiyana, A.; Suryadinata, A.; Annisa, R.; Hakim, A.; Anggraini, W.; Susilowati, R. Activity of inhibit the cell cycle and induct apoptosis in HeLa cancer cell with combination of Sabrang onion (Eleutherine palmifolia (L.) Merr) and Starfruit Mistletoe (Macrosolen cochinchinensis (Lour.) Tiegh). J. Appl. Pharm. Sci. 2018, 8, 122–128. [Google Scholar]
- Rani, V.S. In vitro cytotoxic activity and preliminary phytochemical analysis of the crude extracts of Eleutherine bulbosa (Miller), Urban. World J. Pharm. Res. 2017, 7, 1022–1029. [Google Scholar]
- Lubis, I.A.; Ichwan, M.F.; Mustofa, M.; Satria, D. Anticancer activity of Eleutherine bulbosa (Mill.) Urb. extract on WiDr cell line in vitro. In Proceedings of the 2nd Public Health International Conference (PHICo 2017), Medan, Indonesia, 18–19 December 2017; Atlantis Press: Dordrecht, The Netherlands; pp. 123–127. [Google Scholar]
- Li, X.; Ohtsuki, T.; Koyano, T.; Kowithayakorn, T.; Ishibashi, M. New Wnt/β-Catenin signaling inhibitors isolated from Eleutherine palmifolia. Chem. Asian J. 2009, 4, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Martins, N.; Chaudhary, A.; Garg, N.; Sharma, V.; Kuca, K.; Nepovimova, E.; Tuli, H.S.; Bishayee, A.; Chaudhary, A.; et al. Adjunct use of honey in diabetes mellitus: A consensus or conundrum? Trends Food Sci. Tech. 2020, 106, 254–274. [Google Scholar] [CrossRef]
- Dong, Y.; Fernandes, C.; Liu, Y.; Wu, Y.; Wu, H.; Brophy, M.L.; Deng, L.; Song, K.; Wen, A.; Wong, S.; et al. Role of endoplasmic reticulum stress signalling in diabetic endothelial dysfunction and atherosclerosis. Diab. Vasc. Dis. Res. 2017, 14, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Yuan, B.; Gothai, S.; Arulselvan, P.; Song, X.; Chen, L. Dietary triterpenes in the treatment of type 2 diabetes: To date. Trends Food Sci. Tech. 2018, 72, 34–44. [Google Scholar] [CrossRef]
- Lahrita, L.; Kato, E.; Kawabata, J. Uncovering potential of Indonesian medicinal plants on glucose uptake enhancement and lipid suppression in 3T3-L1 adipocytes. J. Ethnopharm. 2015, 168, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Febrinda, A.E.; Yuliana, N.D.; Ridwan, E.; Wresdiyati, T.; Astawan, M. Hyperglycemic control and diabetes complication preventive activities of Bawang Dayak (Eleutherine palmifolia L. Merr.) bulbs extracts in alloxan-diabetic rats. Int. Food Res. J. 2014, 21, 1405–1411. [Google Scholar]
- Ahmad, I.; Ambarwati, N.S.S.; Indriyanti, N.; Sastyarina, Y.; Rijai, L.; Mun’im, A. Oral glucose tolerance activity of Bawang Dayak (Eleutherine palmifolia L. Merr.) bulbs extract based on the use of different extraction method. Pharmacogn. J. 2018, 10, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Nurcahyawati, D.G.; Plumeriastuti, H.; Maslachah, L. Protection of Dayak Onion tuber extract (Eleutherine palmifolia) against kidney histopathological appearance of albino male rat strain Wistar which was induced by alloxan. KnE Life Sci. 2017, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Fauci, A.S.; Morens, D.M. The perpetual challenge of infectious diseases. N. Engl. J. Med. 2012, 366, 454–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munaeni, W.; Yuhana, M.; Setiawati, M.; Wahyudi, A.T. Effect in white shrimp Litopenaeus vannamei of Eleutherine bulbosa (Mill.) Urb. Powder on immune genes expression and resistance against Vibrio parahaemolyticus infection. Fish Shellfish Immunol. 2020, 102, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Munaeni, W.; Pariakan, A.; Abidin, L.B.; Yuhana, M. In vitro phytochemical and inhibitory potential test of Bawang Hutan bulb extract (Eleutherine palmifolia) on Vibrio harveyi. Microbiol. Indones. 2017, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Man, W.J.; Hou, A.J.; Yang, L.; Xing, X.D.; Yan, M.L.; Guo, X.Y.; Yang, L. The chemical constituents from the active fractions of Eleutherine bulbosa with their anti-microbial activity. Nat. Prod. Res. 2020, 34, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Mahmudah, S.; Muntaha, A.; Muhlisin, A. Effectiveness of Dayak (Eleutherine palmifollia (L) Merr) extracts against Escherichia coli in vitro. Trop. Health Med. Res. 2019, 1, 44–48. [Google Scholar] [CrossRef]
- Sirirak, T.; Syed Musthafa, K.; Lethongkam, S.; Yuenyongsawad, S.; Voravuthikunchai, S.P. Eleutherine americana extract inhibits adherence to and invasion of Caco-2 cells by commonly contaminated Campylobacter spp. in food. J. Food Process. Pres. 2019, 43, e14007. [Google Scholar] [CrossRef]
- Sirirak, T.; Voravuthikunchai, S.P. Eleutherine americana: A candidate for the control of Campylobacter species. Poult. Sci. 2011, 90, 791–796. [Google Scholar] [CrossRef]
- Tan, P.V.; Boda, M.; Sonke, B.; Etoa, F.X.; Nyasse, B. Susceptibility of Helicobacter and Campylobacter to crude extracts prepared from plants used in Cameroonian folk medicine. Pharm. Online 2006, 3, 877–891. [Google Scholar]
- Lee, C.F.; Han, C.K.; Tsau, J.L. In vitro inhibitory activity of Chinese leek extract against Campylobacter species. Int. J. Food Microbiol. 2004, 94, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Harlita, T.D.; Oedjijono, A.A. The antibacterial activity of Dayak onion (Eleutherine palmifolia (L.) merr) towards pathogenic bacteria. Trop. Life Sci. Res. 2018, 29, 39. [Google Scholar] [CrossRef]
- González-Lamothe, R.; Mitchell, G.; Gattuso, M.; Diarra, M.S.; Malouin, F.; Bouarab, K. Plant anti-microbial agents and their effects on plant and human pathogens. Int. J. Mol. Sci. 2009, 10, 3400–3419. [Google Scholar] [CrossRef] [PubMed]
- Padhi, L.; Panda, S.K. Antibacterial activity of Eleutherine bulbosa against multidrug-resistant bacteria. J. Acute Med. 2015, 5, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Garoy, E.Y.; Gebreab, Y.B.; Achila, O.O.; Tekeste, D.G.; Kesete, R.; Ghirmay, R.; Kiflay, R.; Tesfu, T. Methicillin-resistant Staphylococcus aureus (MRSA): Prevalence and anti-microbial sensitivity pattern among patients—A multicenter study in Asmara, Eritrea. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer Science & Business Media: Berlin, Germany, 2008. [Google Scholar]
- Aycicek, H.; Cakiroglu, S.; Stevenson, T.H. Incidence of Staphylococcus aureus in ready-to-eat meals from military cafeterias in Ankara, Turkey. Food Control 2005, 16, 531–534. [Google Scholar] [CrossRef]
- Ifesan, B.O.T.; Hamtasin, C.; Mahabusarakam, W.; Voravuthikunchai, S.P. Inhibitory effect of Eleutherine americana Merr. extract on Staphylococcus aureus isolated from food. J. Food Sci. 2009, 74, M31–M36. [Google Scholar] [CrossRef] [Green Version]
- Ifesan, B.O.; Hamtasin, C.; Mahabusarakam, W.; Voravuthikunchai, S.P. Assessment of antistaphylococcal activity of partially purified fractions and pure compounds from Eleutherine americana. J. Food Prot. 2009, 72, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Limsuwan, S.; Voravuthikunchai, S.P. Boesenbergia pandurata (Roxb.) Schltr., Eleutherine americana Merr. and Rhodomyrtus tomentosa (Aiton) Hassk. as antibiofilm producing and antiquorum sensing in Streptococcus pyogenes. FEMS Immunol. Med. Microbiol. 2008, 53, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Maftuch, M.; Suprastyani, H.; Sanoesi, E.; Farida, N.; Fransira, I.; Habibah, N.; Fatmawati, D.R.; Rinaldi, R.; Nisyak, I.K.; Ardiansyah, D.; et al. Effect of Dayak Onion (Eleutherine palmifolia (L) Merr. crude extract on histopathology of gills, kidney, liver and muscle of Aeromonas hydrophila-infected carp (Cyprinus carpio). Indones. Green Techn. J. 2018, 7, 35–39. [Google Scholar] [CrossRef]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.H.; Mohanta, T.K.; Bae, H. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016, 21, 293. [Google Scholar] [CrossRef]
- Gow, N.A.; Netea, M.G. Medical mycology and fungal immunology: New research perspectives addressing a major world health challenge. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorsaz, S.; Snäkä, T.; Favre-Godal, Q.; Maudens, P.; Boulens, N.; Furrer, P.; Ebrahimi, S.N.; Hamburger, M.; Allémann, E.; Gindro, K.; et al. Identification and mode of action of a plant natural product targeting human fungal pathogens. Antimicrob. Agents Chemother. 2017, 61, e00829-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masfria, M.; Tampubolon, M.S. The Anti-fungal Activity of n-Hexane Extract of Eleutherine palmifolia (L). Merr Bulbs against Candida albicans and Trichophyton mentagrophytes. Open Access Maced. J. Med. Sci. 2019, 7, 3777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cybulska, B.; Herve, M.; Borowski, E.; Gary-Bobo, C.M. Effect of the polar head structure of polyene macrolide anti-fungal antibiotics on the mode of permeabilization of ergosterol-and cholesterol-containing lipidic vesicles studied by 31P-NMR. Mol. Pharm. 1986, 29, 293–298. [Google Scholar]
- Banfi, E.; Scialino, G.; Zampieri, D.; Mamolo, M.G.; Vio, L.; Ferrone, M.; Fermeglia, M.; Paneni, M.S.; Pricl, S. Antifungal and antimycobacterial activity of new imidazole and triazole derivatives. A combined experimental and computational approach. J. Antimicrob. Chemother. 2006, 58, 76–84. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as anti-microbial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayati, S.; Amanah, A.; Indriyati, R. Inhibitory test of Dayak onion (Eleutherine bulbosa Mill.) essential oil to the growth of Malassezia furfur. In Proceedings of the International Conference on Applied Science and Health, August 2019; pp. 247–251. Available online: http://download.garuda.ristekdikti.go.id/article.php?article=1249248&val=13414&title=INHIBITORY%20TEST%20OF%20DAYAK%20ONION%20ELEUTHERINE%20BULBOSA%20MILL%20ESSENTIAL%20OIL%20TO%20THE%20GROWTH%20OF%20MALASSEZIA%20FURFUR (accessed on 10 June 2021).
- Vale, V.V.; Cruz, J.N.; Viana, G.M.; Póvoa, M.M.; Brasil, D.D.; Dolabela, M.F. Naphthoquinones isolated from Eleutherine plicata herb: In vitro antimalarial activity and molecular modeling to investigate their binding modes. Med. Chem. Res. 2020, 3, 487–494. [Google Scholar] [CrossRef]
- Dinarello, C.A. Anti-inflammatory agents: Present and future. Cell 2010, 140, 935–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanh, P.T.B.; Thao, D.T.; Nga, N.T.; Phuong, N.T.; Hung, L.N.; Thien, D.T.; Ha, L.M. Toxicity and anti-inflammatory activities of an extract of the Eleutherine bulbosa rhizome on collagen antibody-induced arthritis in a mouse model. Nat. Prod. Commun. 2018, 13, 883–886. [Google Scholar] [CrossRef] [Green Version]
- Paramita, S.; Nuryanto, M.K. Anti-inflammatory activity of Bawang Dayak (Eleutherine bulbosa (Mill. Urb.)) ethanol bulb extracts. J. Vocation. Health Stud. 2018, 2, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Song, S.H.; Min, H.Y.; Han, A.R.; Nam, J.W.; Seo, E.K.; Park, S.W.; Lee, S.H.; Lee, S.K. Suppression of inducible nitric oxide synthase by (−)-isoeleutherin from the bulbs of Eleutherine americana through the regulation of NF-κB activity. Int. Immunopharmacol. 2009, 9, 298–302. [Google Scholar] [CrossRef]
- Han, A.R.; Min, H.Y.; Nam, J.W.; Lee, N.Y.; Wiryawan, A.; Suprapto, W.; Lee, S.K.; Lee, R.K.; Seo, E.K. Identification of a new naphthalene and its derivatives from the bulb of Eleutherine americana with inhibitory activity on lipopolysaccharide-induced nitric oxide production. Chem. Pharm. Bull. 2008, 56, 1314–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.H.; Yu, E.S.; Han, A.R.; Nam, J.W.; Seo, E.K.; Hwang, E.S. Isoeleutherin and eleutherinol, naturally occurring selective modulators of Th cell-mediated immune responses. Biochem. Biophys. Res. Commun. 2008, 371, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, D.; Ahmed, M.R.; Gomathi, K.; Chitra, K.; Sehgal, P.K.; Jayakumar, R. Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 2004, 25, 1911–1917. [Google Scholar] [CrossRef]
- Rezandaru, F.; Syamsudin, E.; Hadikrishna, I.; Juniantito, V. Effectiveness of the application of Bawang Dayak (Eleutherine palmifolia L. Merr) extracts on healing process in the osteitis alveolar post tooth extraction through fibroblast examination, collagen density and amount of osteogenesis. J. Dentomaxillofacial Sci. 2020, 5, 28–33. [Google Scholar] [CrossRef]
- Upadhyay, A.; Chattopadhyay, P.; Goyary, D.; Mazumder, P.M.; Veer, V. Eleutherine indica L. accelerates in vivo cutaneous wound healing by stimulating Smad-mediated collagen production. J. Ethnopharmacol. 2013, 146, 490–494. [Google Scholar] [CrossRef]
- Qian, W.; Liu, W.; Zhu, D.; Cao, Y.; Tang, A.; Gong, G.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis. Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Park, K.Y.; Kim, J. Synthesis and biological evaluation of the anti-melanogenesis effect of coumaric and caffeic acid-conjugated peptides in human melanocytes. Front. Pharmacol. 2020, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.W.; Jeong, H.O.; Jang, E.J.; Choi, Y.J.; Kim, D.H.; Kim, S.R.; Lee, K.J.; Lee, H.J.; Chun, P.; Byun, Y.; et al. Characterization of a small molecule inhibitor of melanogenesis that inhibits tyrosinase activity and scavenges nitric oxide (NO). Biochim. Biophys. Acta Gen. Sub. 2013, 1830, 4752–4761. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.M.; Chien, Y.C.; Wu, C.H.; Kuo, Y.H.; Wu, W.C.; Pan, Y.Y.; Su, Y.H.; Wen, K.C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. [Google Scholar] [CrossRef]
- Arung, E.T.; Kusuma, I.W.; Christy, E.O.; Shimizu, K.; Kondo, R. Evaluation of medicinal plants from Central Kalimantan for antimelanogenesis. J. Nat. Med. 2009, 63, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Biworo, A.; Abdurrahim, N.N.; Hamidah, S.; Suhartono, E. The effect of Dayak onion (Eleutherine palmifolia (L.) Merr) tuber extract against erythema and melanin index on rat (Rattus norvegicus) skin induced by acute UV. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC.: New York, NY, USA, 2019. [Google Scholar]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munaeni, W.; Widanarni, W.; Yuhana, M.; Setiawati, M.; Wahyudi, A.T. The potential of Buton forest onion Eleutherine bulbosa (Mill.) Urb. extract as a prebiotic and an antioxidant. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Jayanti, N.E.; Harlina; I’tishom, R. An effect of Dayak onion (Eleutherine americana Merr) on quality of the sperm. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC.: New York, NY, USA, 2019; Volume 2108, p. 020035. [Google Scholar]
- Shi, P.; Du, W.; Wang, Y.; Teng, X.; Chen, X.; Ye, L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci. Nutr. 2019, 7, 148–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morabandza, C.J.; Okiemy-Akieli, M.G.; Okiemy, E.; Andzi-Barhé, T.; Ongoka, P.R. Total phenols, total flavonoids content; antioxidant and anti-fungal activities of ethanolic and aqueous extracts of Eleutherine bulbosa (Iridaceae). World J. Pharm. Sci. 2016, 4, 252–255. [Google Scholar]
- Agustin, A.R.; Faika, S.; Ju, Y. Influence of extracting solvents on its antioxidant properties of Bawang Dayak (Eleutherine palmifolia L. Merr). Int. J. Chem. Petrochem. Tech. 2016, 6, 1–10. [Google Scholar]
- Bahtiar, A.; Dewi, R. Antiosteoporosis effects of 70% ethanolic extract combination of Dayak onion bulbs (Eleutherine bulbosa (Mill.) Urb) and Cowpea (Vigna unguiculata (L.) Walp.) on the hypoestrogen rats. Pharmacogn. J. 2019, 11, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Bahtiar, A.; Annisa, R. Effects of Dayak onion Bulbs (Eleutherine bulbosa (Mill.) Urb) on bone development of the hypoestrogen model rat. Pharmacogn. J. 2018, 10, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Purnamasari, A.; Bahtiar, A. Effect of Dayak onion (Eleutherine bulbosa (Mill.) Urb) on uterine wall and lipid profiles of ovariectomized rat. Online J. Bio. Sci. 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Phoem, A.N.; Mayiding, A.; Saedeh, F.; Permpoonpattana, P. Evaluation of Lactobacillus plantarum encapsulated with Eleutherine americana oligosaccharide extract as food additive in yoghurt. Braz. J. Microbiol. 2019, 50, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Phoem, A.N.; Chanthachum, S.; Voravuthikunchai, S.P. Preparation of Eleutherine americana-alginate complex microcapsules and application in Bifidobacterium longum. Nutrients 2015, 7, 831–848. [Google Scholar] [CrossRef]
- Phoem, A.N.; Chanthachum, S.; Voravuthikunchai, S.P. Applications of microencapsulated Bifidobacterium longum with Eleutherine americana in fresh milk tofu and pineapple juice. Nutrients 2015, 7, 2469–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phoem, A.N.; Voravuthikunchai, S.P. Eleutherine americana as a growth promotor for infant intestinal microbiota. Anaerobe 2013, 20, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Damayanti, A.Y.; Choiriyah, N.A.; Naufalina, M.D. Pengaruh penambahan ekstrak bawang dayak (Eleutherine americana Merr.) pada aktivitas antioksidan nuget tempe. Darussalam Nutri. J. 2018, 2, 32–40. [Google Scholar]
- Ifesan, B.O.; Siripongvutikorn, S.; Voravuthikunchai, S.P. Application of Eleutherine americana crude extract in homemade salad dressing. J. Food Protect. 2009, 72, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Ifesan, B.O.; Voravuthikunchai, S.P. Effect of Eleutherine americana Merr. extract on enzymatic activity and enterotoxin production of Staphylococcus aureus in broth and cooked pork. Foodborne Pathog. Dis. 2009, 6, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Ifesan, B.O.T.; Siripongvutikorn, S.; Hutadilok-Towatana, N.; Voravuthikunchai, S.P. Evaluation of the ability of Eleutherine americana crude extract as natural food additive in cooked pork. J. Food Sci. 2009, 74, 352–357. [Google Scholar] [CrossRef]
- Lesmana, H.; Parman, D.H. Utilization of Dayak onion as healthy snacks. Int. J. Nurs. Health Serv. 2019, 2, 397–402. [Google Scholar]
- Ooi, P.S.; Rohaida, A.R.; Nur Hardy, A.D.; Devina, D.; Borhan, A.H.; Kartini, S.; Jupikely, J.S.; Abdul Rahman, M.; Alimon, A.R. Effect of local medicinal herbs as feed additives on production performance and faecal parameters in laying hens. Malays. J. Anim. Sci. 2018, 21, 59–67. [Google Scholar]
- Hardi, D.; Handayani, E. The effects of dietary Eleutherine bulbosa on the growth, leukocyte profile, and digestive enzyme activity of the striped catfish Pangasianodon hypophthalmus. Nusantara Biosci. 2018, 10, 46–51. [Google Scholar]

| Chemical Constituents | Phytochemical Family | IUPAC Names * | Chemical Structures | References |
|---|---|---|---|---|
| Hongconin | Naphthalene | (1R,3R)-5,10-dihydroxy-9-methoxy-1,3-dimethyl-1H-benzo[g]isochromen-4-one |  | [9] |
| Eleutherin | Naphthoquinone | (1R, 3S)-9-methoxy-1,3-dimethyl-3,4-dihydro-1H-benzo[g]isochromene-5,10-dione |  | [17] |
| Eleutherol | Naphthalene | (3R)-4-hydroxy-5-methoxy-3-methyl-3H-benzo[f][2]benzofuran-1-one |  | [17] |
| Eleutherol A | Naphthoquinone | - |  | [21] |
| Eleutherol B | Naphthoquinone | - |  | [21] |
| Eleutherol C | Naphthoquinone | - |  | [21] |
| Eleuthinones B | Naphthoquinone | - |  | [21] |
| Eleuthinones C | Naphthoquinone | - |  | [21] |
| Eleutherinoside A | Naphthalene | 10-hydroxy-2,5-dimethyl-8-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxybenzo[h]chromen-4-one |  | [22] |
| Eleuthoside B | Naphthalene | (3R)-5-methoxy-3-methyl-4 [(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxan-2-yl]oxy-3H-benzo[f][2]benzofuran-1-one |  | [9,16] |
| Elecanacin | Naphthoquinone | 6-Methoxy-2-methyl-1,2,4,4a-tetrahydro-3aH-naphtho[2′,3′:2,3]cyclobuta[1,2-b]furan-5,10-dione |  | [9,16] |
| Eleutherinone | Naphthoquinone | 8-methoxy-1-methyl-1,3-dihydro-naphtho(2,3-c)furan-4,9-dione |  | [23] |
| Isoeleutherin | Naphthoquinone | (1R,3R)-9-methoxy-1,3-dimethyl-3,4-dihydro-1H-benzo[g]isochromene-5,10-dione |  | [23] |
| Eleucanainones A | Naphthoquinone | - |  | [24] |
| Eleucanainones B | Naphthoquinone | - |  | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamarudin, A.A.; Sayuti, N.H.; Saad, N.; Razak, N.A.A.; Esa, N.M. Eleutherine bulbosa (Mill.) Urb. Bulb: Review of the Pharmacological Activities and Its Prospects for Application. Int. J. Mol. Sci. 2021, 22, 6747. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136747
Kamarudin AA, Sayuti NH, Saad N, Razak NAA, Esa NM. Eleutherine bulbosa (Mill.) Urb. Bulb: Review of the Pharmacological Activities and Its Prospects for Application. International Journal of Molecular Sciences. 2021; 22(13):6747. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136747
Chicago/Turabian StyleKamarudin, Ammar Akram, Nor Hafiza Sayuti, Norazalina Saad, Nor Asma Ab. Razak, and Norhaizan Mohd. Esa. 2021. "Eleutherine bulbosa (Mill.) Urb. Bulb: Review of the Pharmacological Activities and Its Prospects for Application" International Journal of Molecular Sciences 22, no. 13: 6747. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22136747