Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics of COVID-19 Patients
2.2. Survival of COVID-19 Patients Is Related to Illness Severity and Hospitalization Days
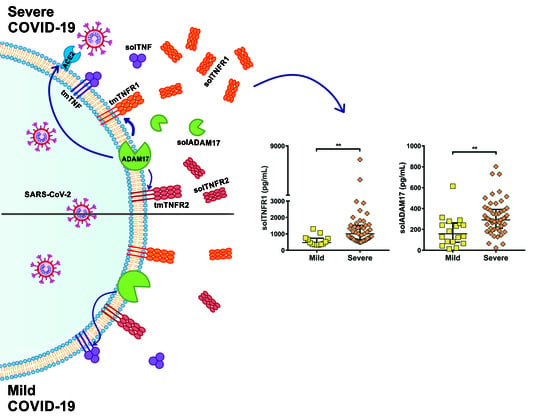
2.3. Soluble Levels of TNFR1 and TNFR2 Are Increased in the Serum of COVID-19 Patients
2.4. TNFR1 and TNFR2 Are Not Increased at the Transcriptional Level in COVID-19 Patients, while ADAM17 Is Increased
2.5. solTIM3 Level, but Not TGF-β, Is Increased in the Serum of COVID-19 Patients
2.6. A High Level of solTNFR1 Is Associated with COVID-19 Deaths and with a Low Level of C-Reactive Protein
2.7. Genetic Variants in TNF, TNFRSF1A, and TNFRSF1B
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Study Design, Population and Samples Collection
4.3. Severity Classification of COVID-19
4.4. Serum and Peripheral Blood Mononuclear Cells Obtention
4.5. ELISA Sandwich Assays
4.6. RNA Extraction and cDNA Synthesis
4.7. Real-Time Quantitative PCR
4.8. Genetic Analysis
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme-2 |
| ADAM10 | A disintegrin and metalloproteinase domain-containing protein 10 |
| ADAM17 | A disintegrin and metalloprotease 17 (also called TACE) |
| ARDS | Acute respiratory distress syndrome |
| COVID-19 | Coronavirus disease 2019 |
| CRP | C-reactive protein |
| ELISA | Enzyme-linked immunosorbent assay |
| HD | Healthy donors |
| HCM | Central Military Hospital |
| HMGB1 | High mobility group box 1 |
| IL | Interleukin |
| INER | National Institute of Respiratory Diseases |
| PBMC | Peripheral blood mononuclear cells |
| qRT-PCR | Real-time reverse transcription polymerase chain reaction assay |
| ROUT | Robust regression and outlier removal method |
| RT-PCR | Real-time reverse transcriptase |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| solTGF-β | Soluble TGF-β |
| solTIM3 | Soluble TIM3 |
| solTNF | Soluble TNF |
| solTNFR1 | Soluble TNFR1 |
| solTNFR2 | Soluble TNFR2 |
| SNPs | Single-nucleotide polymorphisms |
| TACE | TNF-α converting enzyme (also called ADAM17) |
| TGF-β | Transforming growth factor-beta |
| TIM3 | T cell immunoglobulin and mucin domain 3 |
| TMPRSS2 | Transmembrane protease serine 2 |
| tmTNF | Transmembrane TNF |
| TNF | Tumor necrosis factor |
| TNFR1 | TNF receptor 1 |
| TNFR2 | TNF receptor 2 |
References
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A Novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- WHO Coronavirus Disease (COVID-19). Available online: https://covid19.who.int/ (accessed on 10 June 2021).
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ní Choileáin, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the inflammatory response to severe COVID-19 illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Udomsinprasert, W.; Jittikoon, J.; Sangroongruangsri, S.; Chaikledkaew, U. Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for COVID-19: Systematic review with meta-analysis. J. Clin. Immunol. 2021, 41, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Simpson, L.J.; Ferreira, A.-M.; Rustagi, A.; Roque, J.; Asuni, A.; Ranganath, T.; Grant, P.M.; Subramanian, A.; Rosenberg-Hasson, Y.; et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight 2020, 5, e140289. [Google Scholar] [CrossRef] [PubMed]
- Demaret, J.; Lefèvre, G.; Vuotto, F.; Trauet, J.; Duhamel, A.; Labreuche, J.; Varlet, P.; Dendooven, A.; Stabler, S.; Gachet, B.; et al. Severe SARS-CoV-2 Patients Develop a Higher Specific T-Cell Response. Clin. Transl. Immunol. 2020, 9, e1217. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multi-Omics Resolves a Sharp Disease-State Shift Between Mild and Moderate COVID-19. Cell 2020, 183, 1479–1495. [Google Scholar] [CrossRef]
- Lambert, D.W.; Yarski, M.; Warner, F.J.; Thornhill, P.; Parkin, E.T.; Smith, A.I.; Hooper, N.M.; Turner, A.J. Tumor Necrosis Factor-α convertase (ADAM17) mediates regulated ectodomain shedding of the Severe-Scute Respiratory Syndrome-Coronavirus (SARS-CoV) receptor, Angiotensin-Converting Enzyme-2 (ACE2). J. Biol. Chem. 2005, 280, 30113–30119. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, A.; Palacios, Y.; Garcia, I.; Chavez-Galan, L. Transmembrane TNF and Its Receptors TNFR1 and TNFR2 in Mycobacterial Infections. Int. J. Mol. Sci. 2021, 22, 5461. [Google Scholar] [CrossRef]
- Alcami, A. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 2003, 3, 36–50. [Google Scholar] [CrossRef]
- Alejo, A.; Ruiz-Argüello, M.B.; Pontejo, S.M.; Fernández de Marco, M.D.M.; Saraiva, M.; Hernáez, B.; Alcamí, A. Chemokines cooperate with TNF to provide protective anti-viral immunity and to enhance inflammation. Nat. Commun. 2018, 9, 1790. [Google Scholar] [CrossRef] [PubMed]
- Pontejo, S.M.; Sanchez, C.; Ruiz-Argüello, B.; Alcami, A. Insights into ligand binding by a viral tumor necrosis factor (TNF) decoy receptor yield a selective soluble human type 2 TNF receptor. J. Biol. Chem. 2019, 294, 5214–5227. [Google Scholar] [CrossRef] [Green Version]
- Hussell, T.; Pennycook, A.; Openshaw, P.J. Inhibition of Tumor Necrosis Factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 2001, 31, 2566–2573. [Google Scholar] [CrossRef]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of anti-Tumour Necrosis Factor therapy for COVID-19 are urgently needed. Lancet 2020, 395, 1407–1409. [Google Scholar] [CrossRef]
- Atkins, J.L.; Masoli, J.A.H.; Delgado, J.; Pilling, L.C.; Kuo, C.-L.; Kuchel, G.A.; Melzer, D. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Barquera, S.; Rivera, J.A. Obesity in Mexico: Rapid Epidemiological Transition and Food Industry Interference in Health Policies. Lancet Diabetes Endocrinol. 2020, 8, 746–747. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.-K. Gender differences in patients with COVID-19: Focus on severity and mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor Necrosis Factor Signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huovila, A.-P.J.; Turner, A.J.; Pelto-Huikko, M.; Kärkkäinen, I.; Ortiz, R.M. Shedding Light on ADAM Metalloproteinases. Trends Biochem. Sci. 2005, 30, 413–422. [Google Scholar] [CrossRef]
- Gooz, M. ADAM-17: The enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 146–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Xu, P.; Lamouille, S.; Xu, J.; Derynck, R. TACE-mediated ectodomain shedding of the type I TGF-beta receptor downregulates TGF-beta signaling. Mol. Cell 2009, 35, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller-Hackbarth, K.; Dewitz, C.; Schweigert, O.; Trad, A.; Garbers, C.; Rose-John, S.; Scheller, J. A disintegrin and metalloprotease (ADAM) 10 and ADAM17 are major sheddases of T cell immunoglobulin and mucin domain 3 (Tim-3). J. Biol. Chem. 2013, 288, 34529–34544. [Google Scholar] [CrossRef] [Green Version]
- Atzeni, F.; Masala, I.F.; Rodríguez-Carrio, J.; Ríos-Garcés, R.; Gerratana, E.; La Corte, L.; Giallanza, M.; Nucera, V.; Riva, A.; Espinosa, G.; et al. The Rheumatology Drugs for COVID-19 Management: Which and When? J. Clin. Med. 2021, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- NIH COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/ (accessed on 12 May 2021).
- Mortaz, E.; Tabarsi, P.; Jamaati, H.; Dalil Roofchayee, N.; Dezfuli, N.K.; Hashemian, S.M.; Moniri, A.; Marjani, M.; Malekmohammad, M.; Mansouri, D.; et al. Increased Serum Levels of Soluble TNF-α Receptor Is Associated With ICU Mortality in COVID-19 Patients. Front Immunol. 2021, 12, 592727. [Google Scholar] [CrossRef]
- Bowman, E.R.; Cameron, C.M.A.; Avery, A.; Gabriel, J.; Kettelhut, A.; Hecker, M.; Ute Sontich, C.; Tamilselvan, B.; Nichols, C.N.; Richardson, B.; et al. Levels of soluble CD14 and Tumor Necrosis Factor receptors 1 and 2 may be predictive of death in severe coronavirus disease 2019 (COVID-19). J. Infect. Dis. 2021, 223, 805–810. [Google Scholar] [CrossRef]
- Saleh, A.; Sultan, A.; Elashry, M.A.; Farag, A.; Mortada, M.I.; Ghannam, M.A.; Saed, A.M.; Ghoneem, S. Association of TNF-α G-308 a promoter polymorphism with the course and outcome of COVID-19 patients. Immunol. Investig. 2020, 1–12. [Google Scholar] [CrossRef]
- Reséndiz-Hernández, J.M.; Sansores, R.H.; de Jesús Hernández-Zenteno, R.; Vargas-Alarcón, G.; Colín-Barenque, L.; Velázquez-Uncal, M.; Camarena, A.; Ramírez-Venegas, A.; Falfán-Valencia, R. Identification of genetic variants in the TNF promoter associated with COPD secondary to tobacco smoking and its severity. Int. J. Chron. Obstruct. Pulmon. Dis. 2015, 10, 1241–1251. [Google Scholar] [CrossRef] [Green Version]
- Reséndiz-Hernández, J.M.; Ambrocio-Ortiz, E.; Pérez-Rubio, G.; Lopez-Flores, L.A.; Abarca-Rojano, E.; Romero, G.F.P.; Flores-Trujillo, F.; Hernández-Zenteno, R.D.J.; Camarena, A.; Perez-Rodriguez, M.; et al. TNF promoter polymorphisms are associated with genetic susceptibility in COPD secondary to tobacco smoking and biomass burning. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosmicki, J.A.; Horowitz, J.E.; Banerjee, N.; Lanche, R.; Marcketta, A.; Maxwell, E.; Bai, X.; Sun, D.; Backman, J.D.; Sharma, D.; et al. Pan-ancestry exome-wide association analyses of COVID-19 outcomes in 586,157 individuals. Am. J. Hum. Genet. 2021, 108, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D.; et al. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef]
- Smyth, P.; Sessler, T.; Scott, C.J.; Longley, D.B. FLIP(L): The pseudo-caspase. FEBS J. 2020, 287, 4246–4260. [Google Scholar] [CrossRef]
- Choksi, S.; Choudhary, G.; Liu, Z.-G. Transition from TNF-induced inflammation to death signaling. Methods Mol. Biol. 2021, 2248, 73–80. [Google Scholar] [CrossRef]
- Chen, R.; Huang, Y.; Quan, J.; Liu, J.; Wang, H.; Billiar, T.R.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. HMGB1 as a Potential Biomarker and Therapeutic Target for Severe COVID-19. Heliyon 2020, 6, e05672. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed]
- Varchetta, S.; Mele, D.; Oliviero, B.; Mantovani, S.; Ludovisi, S.; Cerino, A.; Bruno, R.; Castelli, A.; Mosconi, M.; Vecchia, M.; et al. Unique immunological profile in patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 604–612. [Google Scholar] [CrossRef]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Allen, R.M.; Carson, W.F.; Schaller, M.; Cavassani, K.A.; Hogaboam, C.M.; Lukacs, N.W.; Matsukawa, A.; Kunkel, S.L. The critical role of Notch ligand delta-like 1 in the pathogenesis of influenza A virus (H1N1) infection. PLoS Pathog. 2011, 7, e1002341. [Google Scholar] [CrossRef] [Green Version]
- Keewan, E.; Beg, S.; Naser, S.A. Anti-TNF-α agents modulate SARS-CoV-2 receptors and increase the risk of infection through Notch-1 signaling. Front. Immunol. 2021, 12, 641295. [Google Scholar] [CrossRef]
- Zipeto, D.; Palmeira, J.D.F.; Argañaraz, G.A.; Argañaraz, E.R. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front. Immunol. 2020, 11, 576745. [Google Scholar] [CrossRef] [PubMed]
- Saheb Sharif-Askari, N.; Saheb Sharif-Askari, F.; Alabed, M.; Temsah, M.-H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol. Ther. Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Giai, C.; Gonzalez, C.; Ledo, C.; Garofalo, A.; Di Genaro, M.S.; Sordelli, D.O.; Gomez, M.I. Shedding of Tumor Necrosis Factor Receptor 1 induced by protein A decreases Tumor Necrosis Factor alpha availability and inflammation during systemic Staphylococcus aureus infection. Infect. Immun. 2013, 81, 4200–4207. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wei, M.; Han, Y.; Zhang, K.; He, L.; Yang, Z.; Su, B.; Zhang, Z.; Hu, Y.; Hui, W. Roles of TNF-alpha gene polymorphisms in the occurrence and progress of SARS-CoV infection: A case-control study. BMC Infect. Dis. 2008, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraudo, C.; Cavaliere, A.; Fichera, G.; Weber, M.; Motta, R.; Pelloso, M.; Tosato, F.; Lupi, A.; Calabrese, F.; Carretta, G.; et al. Validation of a composed COVID-19 chest radiography score: The CARE Project. ERJ Open Res. 2020, 6, 00359-2020. [Google Scholar] [CrossRef]
- Wong, H.Y.F.; Lam, H.Y.S.; Fong, A.H.-T.; Leung, S.T.; Chin, T.W.-Y.; Lo, C.S.Y.; Lui, M.M.-S.; Lee, J.C.Y.; Chiu, K.W.-H.; Chung, T.W.-H.; et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology 2020, 296, E72–E78. [Google Scholar] [CrossRef] [Green Version]
- WHO. COVID-19 Clinical Management. Living Guidance 25 January 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed on 21 July 2021).
- Robinson, P.C.; Richards, D.; Tanner, H.L.; Feldmann, M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020, 2, e653–e655. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Anti-TNF drug adalimumab to be trialled for patients in the community. BMJ 2020, 371, m3847. [Google Scholar] [CrossRef] [PubMed]






| Healthy Donor (HD) n = 25 | Mild n = 17 | Moderate n = 31 | Severe n = 54 | p-Value | |
|---|---|---|---|---|---|
| Age (y) | 33 ± 7 | 44 ± 13 | 53 ± 14 | 51 ± 14 | <0.0001 a,b |
| Gender (M:F) | 7:18 | 10:7 | 21:10 | 39:15 | <0.01 a,b |
| Smoking | 0 | 0 | 2 (6%) | 12 (22%) | <0.05 b |
| Diabetes mellitus | 0 | 1 (6%) | 1 (3%) | 13 (24%) | <0.01 b |
| Systemic Hypertension | 0 | 1 (6%) | 3 (10%) | 6 (11%) | nd |
| Body mass index (kg/m2) | 26 ± 3 | 30 ± 4 | 31 ± 7 | 31 ± 7 | <0.01 b,c |
| HCM/INER ¥ | 18/7 | 17/0 | 23/8 | 17/37 | na |
| Symptom | Mild n = 17 | Moderate n = 31 | Severe n = 54 | p-Value |
|---|---|---|---|---|
| Fever | 10 (59%) | 19 (61%) | 32 (59%) | nd |
| Cough | 10 (59%) | 16 (52%) | 32 (59%) | nd |
| Dyspnea | 2 (11%) | 18 (58%) | 4 (7%) | <0.01 a,b <0.0001 a,c |
| Diarrhea | 2 (11%) | 2 (6%) | 7 (13) | nd |
| Anosmia | 0 | 2 (6%) | 5 (9%) | nd |
| Fatigue | 6 (35%) | 14 (45%) | 31 (57%) | nd |
| Headache | 4 (24%) | 8 (26%) | 19 (35%) | nd |
| Heart rate £ | 91 ± 10 | 106 ± 19 | 106 ± 19 | nd |
| Breathing frequency £ | 20 ± 2 | 22 ± 5 | 27 ± 8 | <0.01 a,c < 0.01 b,c |
| Oxygen saturation £ | 92 ± 4 | 86 ± 13 | 71 ± 19 | <0.0001 a,c <0.01 b,c |
| Parameter | Mild | Moderate | Severe |
|---|---|---|---|
| Leucocytes (RV = 4–10 × 103 cells/mm3) | 8.2 ± 3.9 | 9.7 ± 4.8 | 11 ± 6.1 |
| Lymphocytes (RV = 1–4 × 103 cells/mm3) | 1.8 ± 1.6 | 2.2 ± 4.1 | 1.1 ± 1 |
| C-reactive protein (RV = 1–10 mg/dL) | 81 ±71 | 81 ±71 | 993 ±57 |
| Fibrinogen (RV = 200–400 mg/dL) | 537 ± 224 | 565 ± 245 | 686 ± 223 |
| Glucose (RV = 74–106 mg/dL) | 162 ± 115 | 176 ± 111 | 169 ± 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios, Y.; Ruiz, A.; Ramón-Luing, L.A.; Ocaña-Guzman, R.; Barreto-Rodriguez, O.; Sánchez-Monciváis, A.; Tecuatzi-Cadena, B.; Regalado-García, A.G.; Pineda-Gudiño, R.D.; García-Martínez, A.; et al. Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality. Int. J. Mol. Sci. 2021, 22, 8423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168423
Palacios Y, Ruiz A, Ramón-Luing LA, Ocaña-Guzman R, Barreto-Rodriguez O, Sánchez-Monciváis A, Tecuatzi-Cadena B, Regalado-García AG, Pineda-Gudiño RD, García-Martínez A, et al. Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality. International Journal of Molecular Sciences. 2021; 22(16):8423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168423
Chicago/Turabian StylePalacios, Yadira, Andy Ruiz, Lucero A. Ramón-Luing, Ranferi Ocaña-Guzman, Omar Barreto-Rodriguez, Anahí Sánchez-Monciváis, Brenda Tecuatzi-Cadena, Ana G. Regalado-García, Rey David Pineda-Gudiño, Alicia García-Martínez, and et al. 2021. "Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality" International Journal of Molecular Sciences 22, no. 16: 8423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22168423