Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer
Abstract
:1. Introduction
2. Lipid Rafts
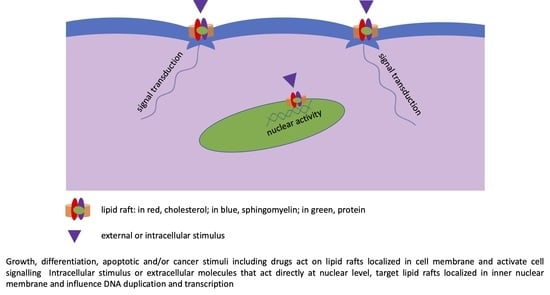
2.1. Lipid Rafts in Cell Membrane
2.2. Lipid Rafts in Nuclear Membrane
3. Sphingolipids in Cancer
3.1. Sphingomyelin
3.2. Ceramide
3.3. Ceramide-1-Phosphate
3.4. Sphingosine-1-Phosphate
3.5. Gangliosides
4. Cholesterol in Cancer
5. Lipid Rafts in Cancer
5.1. Lipid Rafts in Metastasis
5.2. Lipid Rafts as Therapeutic Targets in Cancer
5.2.1. Liquid Tumors
5.2.2. Solid Tumors
5.2.3. Breast Cancer
5.2.4. Gastric Cancer
5.2.5. Liver Cancer
5.2.6. Pancreatic Cancer
5.2.7. Others
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C1P | Ceramide 1 phosphate |
| CerK | Ceramide kinase |
| GD | Disialoganglioside |
| FADD DISC | Fas-associated protein with death domain, death-inducing signaling complex |
| GM | Monosialoganglioside |
| S1P | Sphingosine 1 phosphate |
| S1PR | Sphingosine-1-phosphate receptor |
| SphK | Sphingosine kinase |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
References
- Fantini, J.; Garmy, N.; Mahfoud, R.; Yahi, N. Lipid rafts: Structure, function and role in HIV, Alzheimer’s and prion diseases. Expert Rev. Mol. Med. 2002, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Okazaki, T. Ceramide/Sphingomyelin Rheostat Regulated by Sphingomyelin Synthases and Chronic Diseases in Murine Models. J. Lipid Atheroscler. 2020, 9, 380–405. [Google Scholar] [CrossRef] [PubMed]
- Grassi, S.; Giussani, P.; Mauri, L.; Prioni, S.; Sonnino, S.; Prinetti, A. Lipid rafts and neurodegeneration: Structural and functional roles in physiologic aging and neurodegenerative diseases. J. Lipid Res. 2020, 61, 636–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipione, S.; Monyror, J.; Galleguillos, D.; Steinberg, N.; Kadam, V. Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications. Front. Neurosci. 2020, 14, 572965. [Google Scholar] [CrossRef] [PubMed]
- Rudajev, V.; Novotný, J. The Role of Lipid Environment in Ganglioside GM1-Induced Amyloid β Aggregation. Membranes 2020, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Chen, W.-L. Host Lipid Rafts as the Gates for Listeria monocytogenes Infection: A Mini-Review. Front. Immunol. 2020, 11, 1666. [Google Scholar] [CrossRef]
- Fecchi, K.; Anticoli, S.; Peruzzu, D.; Iessi, E.; Gagliardi, M.C.; Matarrese, P.; Ruggieri, A. Coronavirus Interplay With Lipid Rafts and Autophagy Unveils Promising Therapeutic Targets. Front. Microbiol. 2020, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Filippini, A.; D’Alessio, A. Caveolae and Lipid Rafts in Endothelium: Valuable Organelles for Multiple Functions. Biomolecules 2020, 10, 1218. [Google Scholar] [CrossRef]
- Tomassoni, M.L.; Albi, E.; Magni, M.V. Changes of nuclear membrane fluidity during rat liver regeneration. Biochem. Mol. Biol. Int. 1999, 47, 1049–1059. [Google Scholar] [CrossRef]
- Heessen, S.; Fornerod, M. The inner nuclear envelope as a transcription factor resting place. EMBO Rep. 2007, 8, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Cascianelli, G.; Villani, M.; Tosti, M.; Marini, F.; Bartoccini, E.; Magni, M.V.; Albi, E. Lipid Microdomains in Cell Nucleus. Mol. Biol. Cell 2008, 19, 5289–5295. [Google Scholar] [CrossRef] [Green Version]
- Scassellati, C.; Albi, E.; Cmarko, D.; Tiberi, C.; Cmarkova, J.; Bouchet-Marquis, C.; Verschure, P.J.; Driel, R.; Magni, M.V.; Fakan, S. Intranuclear sphingomyelin is associated with transcriptionally active chromatin and plays a role in nuclear integrity. Biol. Cell 2010, 102, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, M.; Albi, E. Nuclear Lipids in the Nervous System: What they do in Health and Disease. Neurochem. Res. 2016, 42, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Conte, C.; Arcuri, C.; Cataldi, S.; Mecca, C.; Codini, M.; Ceccarini, M.R.; Patria, F.; Beccari, T.; Albi, E. Niemann-Pick Type A Disease: Behavior of Neutral Sphingomyelinase and Vitamin D Receptor. Int. J. Mol. Sci. 2019, 20, 2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albi, E.; Alessenko, A.; Grösch, S. Sphingolipids in Inflammation. Mediat. Inflamm. 2018, 2018, 7464702. [Google Scholar] [CrossRef] [Green Version]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef]
- Li, Z.; Guan, M.; Lin, Y.; Cui, X.; Zhang, Y.; Zhao, Z.; Zhu, J. Aberrant Lipid Metabolism in Hepatocellular Carcinoma Revealed by Liver Lipidomics. Int. J. Mol. Sci. 2017, 18, 2550. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, G.; Moorthi, S.; Luberto, C. Role and function of sphingomyelin biosynthesis in the development of cancer. Adv. Cancer Res. 2018, 140, 61–96. [Google Scholar]
- Burns, T.A.; Subathra, M.; Signorelli, P.; Choi, Y.; Yang, X.; Wang, Y.; Villani, M.; Bhalla, K.; Zhou, D.; Luberto, C. Sphingomyelin synthase 1 activity is regulated by theBCR-ABLoncogene. J. Lipid Res. 2013, 54, 794–805. [Google Scholar] [CrossRef] [Green Version]
- Moorthi, S.; Burns, T.A.; Yu, G.Q.; Luberto, C. Bcr-Abl regulation of sphingomyelin synthase 1 reveals a novel oncogenic-driven mechanism of protein up-regulation. FASEB J. 2018, 32, 4270–4283. [Google Scholar] [CrossRef] [Green Version]
- Van Der Hoeven, D.; Cho, K.-J.; Zhou, Y.; Ma, X.; Chen, W.; Naji, A.; Montufar-Solis, D.; Zuo, Y.; Kovar, S.E.; Levental, K.R.; et al. Sphingomyelin Metabolism Is a Regulator of K-Ras Function. Mol. Cell. Biol. 2017, 38, e00373-17. [Google Scholar] [CrossRef] [Green Version]
- Albi, E.; Cataldi, S.; Ceccarini, M.R.; Conte, C.; Ferri, I.; Fettucciari, K.; Patria, F.; Beccari, T.; Codini, M. Gentamicin Targets Acid Sphingomyelinase in Cancer: The Case of the Human Gastric Cancer NCI-N87 Cells. Int. J. Mol. Sci. 2019, 20, 4375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albi, E.; Cataldi, S.; Ferri, I.; Sidoni, A.; Traina, G.; Fettucciari, K.; Ambesi-Impiombato, F.S.; Lazzarini, A.; Curcio, F.; Ceccarini, M.R.; et al. VDR independent induction of acid-sphingomyelinase by 1,23(OH)2 D3 in gastric cancer cells: Impact on apoptosis and cell morphology. Biochimie 2018, 146, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Arcuri, C.; Lazzarini, A.; Nakashidze, I.; Ragonese, F.; Fioretti, B.; Ferri, I.; Conte, C.; Codini, M.; Beccari, T.; et al. Effect of 1α,25(OH)2 Vitamin D3 in Mutant P53 Glioblastoma Cells: Involvement of Neutral Sphingomyelinase1. Cancers 2020, 12, 3163. [Google Scholar] [CrossRef] [PubMed]
- Beckham, T.H.; Cheng, J.C.; Marrison, S.T.; Norris, J.S.; Liu, X. Interdiction of Sphingolipid Metabolism to Improve Standard Cancer Therapies. Adv. Cancer Res. 2013, 117, 1–36. [Google Scholar] [PubMed] [Green Version]
- Sassa, T.; Suto, S.; Okayasu, Y.; Kihara, A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta 2012, 1821, 1031–1037. [Google Scholar] [CrossRef] [Green Version]
- Senkal, C.E.; Ponnusamy, S.; Bielawski, J.; Hannun, Y.A.; Ogretmen, B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010, 24, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Schiffmann, S.; Ziebell, S.; Sandner, J.; Birod, K.; Deckmann, K.; Hartmann, D.; Rode, S.; Schmidt, H.; Angioni, C.; Geisslinger, G.; et al. Activation of ceramide synthase 6 by celecoxib leads to a selective induction of C16:0-ceramide. Biochem. Pharmacol. 2010, 80, 1632–1640. [Google Scholar] [CrossRef] [Green Version]
- Verlekar, D.; Wei, S.-J.; Cho, H.E.; Yang, S.; Kang, M.H. Ceramide synthase-6 confers resistance to chemotherapy by binding to CD95/Fas in T-cell acute lymphoblastic leukemia. Cell Death Dis. 2018, 9, 925. [Google Scholar] [CrossRef]
- Moro, K.; Kawaguchi, T.; Tsuchida, J.; Gabriel, E.; Qi, Q.; Yan, L.; Wakai, T.; Takabe, K.; Nagahashi, M. Ceramide species are elevated in human breast cancer and are associated with less aggressiveness. Oncotarget 2018, 9, 19874–19890. [Google Scholar] [CrossRef] [Green Version]
- Elojeimy, S.; Liu, X.; McKillop, J.C.; El-Zawahry, A.M.; Holman, D.H.; Cheng, J.Y.; Meacham, W.D.; Mahdy, A.E.; Saad, A.F.; Turner, L.S.; et al. Role of Acid Ceramidase in Resistance to FasL: Therapeutic Approaches Based on Acid Ceramidase Inhibitors and FasL Gene Therapy. Mol. Ther. 2007, 15, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.; Meca-Cortés, Ó.; Abad, J.L.; García, S.; Rubio, N.; Díaz, A.; Celià-Terrassa, T.; Cingolani, F.; Bermudo, R.; Fernández, P.L.; et al. Acid ceramidase as a therapeutic target in metastatic prostate cancer. J. Lipid Res. 2013, 54, 1207–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.C.; Bai, A.; Beckham, T.H.; Marrison, S.T.; Yount, C.L.; Young, K.; Lu, P.; Bartlett, A.M.; Wu, B.X.; Keane, B.J.; et al. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J. Clin. Investig. 2013, 123, 4344–4358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckham, T.H.; Cheng, J.C.; Lu, P.; Marrison, S.T.; Norris, J.S.; Liu, X. Acid ceramidase promotes nuclear export of PTEN through sphingosine 1-phosphate mediated Akt signaling. PLoS ONE 2013, 8, e76593. [Google Scholar] [CrossRef] [PubMed]
- Sänger, N.; Ruckhäberle, E.; Györffy, B.; Engels, K.; Heinrich, T.; Fehm, T.; Graf, A.; Holtrich, U.; Becker, S.; Karn, T. Acid ceramidase is associated with an improved prognosis in both DCIS and invasive breast cancer. Mol. Oncol. 2014, 9, 58–67. [Google Scholar] [CrossRef]
- Hanker, L.; Karn, T.; Holtrich, U.; Gätje, R.; Rody, A.; Heinrich, T.; Ruckhäberle, E.; Engels, K. Acid Ceramidase (AC)—A Key Enzyme of Sphingolipid Metabolism—Correlates With Better Prognosis in Epithelial Ovarian Cancer. Int. J. Gynecol. Pathol. 2013, 32, 249–257. [Google Scholar] [CrossRef]
- Presa, N.; Gomez-Larrauri, A.; Dominguez-Herrera, A.; Trueba, M.; Gomez-Muñoz, A. Novel signaling aspects of ceramide 1-phosphate. Biochim. Biophys. Acta 2020, 1865, 158630. [Google Scholar] [CrossRef]
- Newcomb, B.; Rhein, C.; Mileva, I.; Ahmad, R.; Clarke, C.J.; Snider, J.; Obeid, L.M.; Hannun, Y.A. Identification of an acid sphingomyelinase ceramide kinase pathway in the regulation of the chemokine CCL5. J. Lipid Res. 2018, 59, 1219–1229. [Google Scholar] [CrossRef] [Green Version]
- Rivera, I.-G.; Ordoñez, M.; Presa, N.; Gangoiti, P.; Gomez-Larrauri, A.; Trueba, M.; Fox, T.; Kester, M.; Gómez-Muñoz, A. Ceramide 1-phosphate regulates cell migration and invasion of human pancreatic cancer cells. Biochem. Pharmacol. 2016, 102, 107–119. [Google Scholar] [CrossRef]
- Bini, F.; Frati, A.; Garcia-Gil, M.; Battistini, C.; Granado, M.; Martinesi, M.; Mainardi, M.; Vannini, E.; Luzzati, F.; Caleo, M.; et al. New signalling pathway involved in the anti-proliferative action of vitamin D3 and its analogues in human neuroblastoma cells. A role for ceramide kinase. Neuropharmacology 2012, 63, 524–537. [Google Scholar] [CrossRef]
- Pastukhov, O.; Schwalm, S.; Zangemeister-Wittke, U.; Fabbro, D.; Bornancin, F.; Japtok, L.; Kleuser, B.; Pfeilschifter, J.; Huwiler, A. The ceramide kinase inhibitor NVP-231 inhibits breast and lung cancer cell proliferation by inducing M phase arrest and subsequent cell death. Br. J. Pharmacol. 2014, 171, 5829–5844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadi, L.A.; Calcaterra, F.; Brambilla, L.; Carenza, C.; Marfia, G.; Della Bella, S.; Riboni, L. Enhanced phosphorylation of sphingosine and ceramide sustains the exuberant proliferation of endothelial progenitors in Kaposi sarcoma. J. Leukoc. Biol. 2018, 103, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, S.; Erhardt, M.; Römer, I.; Pfeilschifter, J.; Zangemeister-Wittke, U.; Huwiler, A. Ceramide Kinase Is Upregulated in Metastatic Breast Cancer Cells and Contributes to Migration and Invasion by Activation of PI 3-Kinase and Akt. Int. J. Mol. Sci. 2020, 21, 1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomizawa, S.; Tamori, M.; Tanaka, A.; Utsumi, N.; Sato, H.; Hatakeyama, H.; Hisaka, A.; Kohama, T.; Yamagata, K.; Honda, T.; et al. Inhibitory effects of ceramide kinase on Rac1 activation, lamellipodium formation, cell migration, and metastasis of A549 lung cancer cells. Biochim. Biophys. Acta 2020, 1865, 158675. [Google Scholar] [CrossRef] [PubMed]
- Pyne, N.J.; Pyne, S. Recent advances in the role of sphingosine 1-phosphate in cancer. FEBS Lett. 2020, 594, 3583–3601. [Google Scholar] [CrossRef]
- Moro, K.; Nagahashi, M.; Gabriel, E.; Takabe, K.; Wakai, T. Clinical application of ceramide in cancer treatment. Breast Cancer 2019, 26, 407–415. [Google Scholar] [CrossRef]
- Knapp, P.; Chomicz, K.; Świderska, M.; Chabowski, A.; Jach, R. Unique Roles of Sphingolipids in Selected Malignant and Nonmalignant Lesions of Female Reproductive System. BioMed Res. Int. 2019, 2019, 4376583. [Google Scholar] [CrossRef] [Green Version]
- Sukocheva, O.A.; Furuya, H.; Ng, M.L.; Friedemann, M.; Menschikowski, M.; Tarasov, V.V.; Chubarev, V.N.; Klochkov, S.G.; Neganova, M.E.; Mangoni, A.A.; et al. Sphingosine kinase and sphingosine-1-phosphate receptor signaling pathway in inflammatory gastrointestinal disease and cancers: A novel therapeutic target. Pharmacol. Ther. 2020, 207, 107464. [Google Scholar] [CrossRef]
- Maceyka, M.; Rohrbach, T.; Milstien, S.; Spiegel, S. Role of Sphingosine Kinase 1 and Sphingosine-1-Phosphate Axis in Hepatocellular Carcinoma. Handb. Exp. Pharmacol. 2020, 259, 3–17. [Google Scholar]
- Carrié, L.; Virazels, M.; Dufau, C.; Montfort, A.; Levade, T.; Ségui, B.; Andrieu-Abadie, N. New Insights into the Role of Sphingolipid Metabolism in Melanoma. Cells 2020, 9, 1967. [Google Scholar] [CrossRef]
- Hawkins, C.C.; Ali, T.; Ramanadham, S.; Hjelmeland, A.B. Sphingolipid Metabolism in Glioblastoma and Metastatic Brain Tumors: A Review of Sphingomyelinases and Sphingosine-1-Phosphate. Biomolecules 2020, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, J.; Nagahashi, M.; Nakajima, M.; Moro, K.; Tatsuda, K.; Ramanathan, R.; Takabe, K.; Wakai, T. Breast cancer sphingosine-1-phosphate is associated with phospho-sphingosine kinase 1 and lymphatic metastasis. J. Surg. Res. 2016, 205, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagahashi, M.; Ramachandran, S.; Kim, E.Y.; Allegood, J.C.; Rashid, O.M.; Yamada, A.; Zhao, R.; Milstien, S.; Zhou, H.; Spiegel, S.; et al. Sphingosine-1-Phosphate Produced by Sphingosine Kinase 1 Promotes Breast Cancer Progression by Stimulating Angiogenesis and Lymphangiogenesis. Cancer Res. 2012, 72, 726–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, J.; Proia, R.L.; Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008, 8, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Olesch, C.; Sirait-Fischer, E.; Berkefeld, M.; Fink, A.F.; Susen, R.M.; Ritter, B.; Michels, B.E.; Steinhilber, D.; Greten, F.R.; Savai, R.; et al. S1PR4 ablation reduces tumor growth and improves chemotherapy via CD8+ T cell expansion. J. Clin. Investig. 2020, 5461–5476. [Google Scholar] [CrossRef]
- Cavdarli, S.; Delannoy, P.; Groux-Degroote, S. O-acetylated Gangliosides as Targets for Cancer Immunotherapy. Cells 2020, 9, 741. [Google Scholar] [CrossRef] [Green Version]
- Cavdarli, S.; Groux-Degroote, S.; Delannoy, P. Gangliosides: The Double-Edge Sword of Neuro-Ectodermal Derived Tumors. Biomolecules 2019, 9, 311. [Google Scholar] [CrossRef] [Green Version]
- Iwasawa, T.; Zhang, P.; Ohkawa, Y.; Momota, H.; Wakabayashi, T.; Ohmi, Y.; Bhuiyan, R.H.; Furukawa, K.; Furukawa, K. Enhancement of malignant properties of human glioma cells by ganglioside GD3/GD2. Int. J. Oncol. 2018, 52, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Ohmi, Y.; Kambe, M.; Ohkawa, Y.; Hamamura, K.; Tajima, O.; Takeuchi, R.; Furukawa, K.; Furukawa, K. Differential roles of gangliosides in malignant properties of melanomas. PLoS ONE 2018, 13, e0206881. [Google Scholar] [CrossRef] [Green Version]
- Schengrund, C.-L. Gangliosides and Neuroblastomas. Int. J. Mol. Sci. 2020, 21, 5313. [Google Scholar] [CrossRef]
- Sait, S.; Modak, S. Anti-GD2 immunotherapy for neuroblastoma. Exp. Rev. Anticancer Ther. 2017, 17, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Labrada, M.; Dorvignit, D.; Hevia, G.; Rodríguez, N.; Hernández, A.M.; Vázquez, A.M.; Fernández, L.E. GM3(Neu5Gc) ganglioside: An evolution fixed neoantigen for cancer immunotherapy. Semin. Oncol. 2018, 45, 41–51. [Google Scholar] [CrossRef]
- Zheng, C.; Terreni, M.; Sollogoub, M.; Zhang, Y. Ganglioside GM3 and Its Role in Cancer. Curr. Med. Chem. 2019, 26, 2933–2947. [Google Scholar] [CrossRef] [PubMed]
- Borgquist, S.; Butt, T.; Almgren, P.; Shiffman, D.; Stocks, T.; Orho-Melander, M.; Manjer, J.; Melander, O. Apolipoproteins, lipids and risk of cancer. Int. J. Cancer 2016, 138, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.-H.; Yang, Z.-S.; Li, M.; Chen, Y.; Zhao, X.-F.; Qin, Y.-Y.; Song, J.-Q.; Wang, B.-B.; Yuan, B.; Cui, X.-L.; et al. High Serum Levels of Cholesterol Increase Antitumor Functions of Nature Killer Cells and Reduce Growth of Liver Tumors in Mice. Gastroenterology 2020, 158, 1713–1727. [Google Scholar] [CrossRef]
- Gao, R.; Liang, J.-H.; Wang, L.; Zhu, H.-Y.; Wu, W.; Cao, L.; Fan, L.; Li, J.; Yang, T.; Xu, W. Low serum cholesterol levels predict inferior prognosis and improve NCCN-IPI scoring in diffuse large B cell lymphoma. Int. J. Cancer 2018, 143, 1884–1895. [Google Scholar] [CrossRef]
- Lin, X.; Liu, L.; Fu, Y.; Gao, J.; He, Y.; Wu, Y.; Lian, X. Dietary Cholesterol Intake and Risk of Lung Cancer: A Meta-Analysis. Nutrients 2018, 10, 185. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Jeong, S.; Wu, M.; Jin, Z.; Zhou, J.-Y.; Han, R.-Q.; Yang, J.; Zhang, X.-F.; Wang, X.-S.; Liu, A.-M.; et al. Dietary Intake of Fatty Acids, Total Cholesterol, and Stomach Cancer in a Chinese Population. Nutrients 2019, 11, 1730. [Google Scholar] [CrossRef] [Green Version]
- Freed-Pastor, W.A.; Mizuno, H.; Zhao, X.; Langerød, A.; Moon, S.-H.; Rodriguez-Barrueco, R.; Barsotti, A.M.; Chicas, A.; Li, W.; Polotskaia, A.; et al. Mutant p53 Disrupts Mammary Tissue Architecture via the Mevalonate Pathway. Cell 2012, 148, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Sorrentino, G.; Ruggeri, N.; Specchia, V.; Cordenonsi, M.; Mano, M.; Dupont, S.; Manfrin, A.; Ingallina, E.; Sommaggio, R.; Piazza, S.; et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 2014, 16, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Yang, E.J.; Shim, J.S. Cholesterol Trafficking: An Emerging Therapeutic Target for Angiogenesis and Cancer. Cells 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Riscal, R.; Skuli, N.; Simon, M.C. Even Cancer Cells Watch Their Cholesterol! Mol. Cell 2019, 76, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.J.; Yu, R.; Longo, J.; Archer, M.C.; Penn, L.Z. The interplay between cell signalling and the mevalonate pathway in cancer. Nat. Rev. Cancer 2016, 16, 718–731. [Google Scholar] [CrossRef]
- Bathaie, S.Z.; Ashrafi, M.; Azizian, M.; Tamanoi, F. Mevalonate Pathway and Human Cancers. Curr. Mol. Pharmacol. 2017, 10, 77–85. [Google Scholar]
- Clendening, J.W.; Penn, L.Z. Targeting tumor cell metabolism with statins. Oncogene 2012, 31, 4967–4978. [Google Scholar] [CrossRef] [Green Version]
- McDougall, J.A.; Malone, K.E.; Daling, J.R.; Cushing-Haugen, K.L.; Porter, P.L.; Li, C.I. Long-Term Statin Use and Risk of Ductal and Lobular Breast Cancer among Women 55 to 74 Years of Age. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1529–1537. [Google Scholar] [CrossRef] [Green Version]
- Aylon, Y.; Oren, M. The Hippo pathway, p53 and cholesterol. Cell Cycle 2016, 15, 2248–2255. [Google Scholar] [CrossRef]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.-H.; Huang, C.-H.; Houlihan, S.L.; Regunath, K.; Freed-Pastor, W.A.; Morris, J.P.; Tschaharganeh, D.F.; Kastenhuber, E.R.; Barsotti, A.M.; Culp-Hill, R.; et al. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell 2019, 176, 564–580.e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furth, N.; Aylon, Y.; Oren, M. p53 shades of Hippo. Cell Death Differ. 2018, 25, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Song, B.-L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimento, A.; Casaburi, I.; Avena, P.; Trotta, F.; De Luca, A.; Rago, V.; Pezzi, V.; Sirianni, R. Cholesterol and Its Metabolites in Tumor Growth: Therapeutic Potential of Statins in Cancer Treatment. Front. Endocrinol. 2019, 9, 807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filardo, E.J. A role for G-protein coupled estrogen receptor (GPER) in estrogen-induced carcinogenesis: Dysregulated glandular homeostasis, survival and metastasis. J. Steroid Biochem. Mol. Biol. 2018, 176, 38–48. [Google Scholar] [CrossRef]
- Silvente-Poirot, S.; Dalenc, F.; Poirot, M. The Effects of Cholesterol-Derived Oncometabolites on Nuclear Receptor Function in Cancer. Cancer Res. 2018, 78, 4803–4808. [Google Scholar] [CrossRef] [Green Version]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Greenlee, J.D.; Subramanian, T.; Liu, K.; King, M.R. Rafting Down the Metastatic Cascade: The Role of Lipid Rafts in Cancer Metastasis, Cell Death, and Clinical Outcomes. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy. J. Lipid Res. 2020, 61, 611–635. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.D.; Inder, K.L.; Shah, A.K.; Cristino, A.S.; McKie, A.B.; Gabra, H.; Davis, M.J.; Hill, M.M. Integrative Analysis of Subcellular Quantitative Proteomics Studies Reveals Functional Cytoskeleton Membrane–Lipid Raft Interactions in Cancer. J. Proteome Res. 2016, 15, 3451–3462. [Google Scholar] [CrossRef]
- Tuzmen, S.; Hostetter, G.; Watanabe, A.; Ekmekçi, C.; Carrigan, E.P.; Shechter, I.; Kallioniemi, O.; Miller, L.J.; Mousses, S. Characterization of farnesyl diphosphate farnesyl transferase 1 (FDFT1) expression in cancer. Pers. Med. 2019, 16, 51–65. [Google Scholar] [CrossRef]
- Goossens, P.; Rodriguez-Vita, J.; Etzerodt, A.; Masse, M.; Rastoin, O.; Gouirand, V.; Ulas, T.; Papantonopoulou, O.; Van Eck, M.; Auphan-Anezin, N.; et al. Membrane Cholesterol Efflux Drives Tumor-Associated Macrophage Reprogramming and Tumor Progression. Cell Metab. 2019, 29, 1376–1389.e4. [Google Scholar] [CrossRef] [Green Version]
- Hengst, J.A.; Guilford, J.M.; Fox, T.E.; Wang, X.; Conroy, E.J.; Yun, J.K. Sphingosine kinase 1 localized to the plasma membrane lipid raft microdomain overcomes serum deprivation induced growth inhibition. Arch. Biochem. Biophys. 2009, 492, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Pitson, S.M. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 2011, 36, 97–107. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, S.-H.; Cho, H.-Y.; Shin, H.-S.; Sung, H.-R.; Jung, J.-R.; Quan, M.-L.; Jiang, D.-H.; Bae, H.-R. GD3 Accumulation in Cell Surface Lipid Rafts Prior to Mitochondrial Targeting Contributes to Amyloid-β-induced Apoptosis. J. Korean Med Sci. 2010, 25, 1492–1498. [Google Scholar] [CrossRef] [Green Version]
- Sorice, M.; Matarrese, P.; Tinari, A.; Giammarioli, A.M.; Garofalo, T.; Manganelli, V.; Ciarlo, L.G.; Gambardella, L.; Maccari, G.; Botta, M.; et al. Raft component GD3 associates with tubulin following CD95/Fas ligation. FASEB J. 2009, 23, 3298–3308. [Google Scholar] [CrossRef]
- Garofalo, T.; Tinari, A.; Matarrese, P.; Giammarioli, A.M.; Manganelli, V.; Ciarlo, L.; Misasi, R.; Sorice, M.; Malorni, W. Do mitochondria act as “cargo boats” in the journey of GD3 to the nucleus during apoptosis? FEBS Lett. 2007, 581, 3899–3903. [Google Scholar] [CrossRef] [Green Version]
- Preta, G. New Insights Into Targeting Membrane Lipids for Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 571237. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; He, X.; He, Z.; Wang, T.; Wan, L.; Chen, L.; Yan, N. Hydroxypropyl-β-cyclodextrin attenuates the epithelial-to-mesenchymal transition via endoplasmic reticulum stress in MDA-MB-231 breast cancer cells. Mol. Med. Rep. 2019, 21, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Song, Y.; Qu, J.; Che, X.; Song, N.; Fan, Y.; Wen, T.; Xu, L.; Gong, J.; Wang, X.; et al. The Chemokine Receptor CXCR4 and c-MET Cooperatively Promote Epithelial-Mesenchymal Transition in Gastric Cancer Cells. Transl. Oncol. 2018, 11, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Muñoz, B.; Yurrita, M.M.; Martín-Villar, E.; Carrasco-Ramírez, P.; Megías, D.; Renart, J.; Quintanilla, M. The transmembrane domain of podoplanin is required for its association with lipid rafts and the induction of epithelial-mesenchymal transition. Int. J. Biochem. Cell Biol. 2011, 43, 886–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, W.; Hao, Z.; Han, J.-L.; Zhu, D.-J.; Jin, Z.-F.; Xie, W. CAV-1 contributes to bladder cancer progression by inducing epithelial-to-mesenchymal transition. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Gauthier-Rouvière, C.; Bodin, S.; Comunale, F.; Planchon, D. Flotillin membrane domains in cancer. Cancer Metastasis Rev. 2020, 39, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Peng, J.; Li, X.; Leng, A.; Liu, T. miR-449a targets Flot2 and inhibits gastric cancer invasion by inhibiting TGF-β-mediated EMT. Diagn. Pathol. 2015, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Niu, M.; Yuan, X.; Wu, K.; Liu, A. CD44 as a tumor biomarker and therapeutic target. Exp. Hematol. Oncol. 2020, 9, 36. [Google Scholar] [CrossRef]
- Murai, T.; Maruyama, Y.; Mio, K.; Nishiyama, H.; Suga, M.; Sato, C. Low Cholesterol Triggers Membrane Microdomain-dependent CD44 Shedding and Suppresses Tumor Cell Migration. J. Biol. Chem. 2011, 286, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Babina, I.S.; McSherry, A.E.; Donatello, S.; Hill, A.D.K.; Hopkins, A.M. A novel mechanism of regulating breast cancer cell migration via palmitoylation-dependent alterations in the lipid raft affiliation of CD44. Breast Cancer Res. 2014, 16, R19. [Google Scholar] [CrossRef] [Green Version]
- Chantôme, A.; Potier-Cartereau, M.; Clarysse, L.; Fromont, G.; Marionneau-Lambot, S.; Guéguinou, M.; Pagès, J.-C.; Collin, C.; Oullier, T.; Girault, A.; et al. Pivotal Role of the Lipid Raft SK3–Orai1 Complex in Human Cancer Cell Migration and Bone Metastases. Cancer Res. 2013, 73, 4852–4861. [Google Scholar] [CrossRef] [Green Version]
- Tisza, M.J.; Zhao, W.; Fuentes, J.S.R.; Prijic, S.; Chen, X.; Levental, I.; Chang, J.T. Motility and stem cell properties induced by the epithelial-mesenchymal transition require destabilization of lipid rafts. Oncotarget 2016, 7, 51553–51568. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, E.M.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. 2015, 240, 760–773. [Google Scholar] [CrossRef] [Green Version]
- Gajate, C.; Mollinedo, F. Lipid Rafts, Endoplasmic Reticulum and Mitochondria in the Antitumor Action of the Alkylphospholipid Analog Edelfosine. Anti-Cancer Agents Med. Chem. 2014, 14, 509–527. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, C.; Capasso, A. Role of lipid rafts/caveolae in the anticancer effect of endocannabinoids. Mini Rev. Med. Chem. 2012, 12, 1119–1126. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Lipid rafts and clusters of apoptotic signaling molecule-enriched rafts in cancer therapy. Futur. Oncol. 2010, 6, 811–821. [Google Scholar] [CrossRef]
- Lavie, Y.; Liscovitch, M. Changes in lipid and protein constituents of rafts and caveolae in multidrug resistant cancer cells and their functional consequences. Glycoconj. J. 2001, 17, 253–259. [Google Scholar] [CrossRef]
- Gajate, C.; Mollinedo, F. Lipid raft-mediated Fas/CD95 apoptotic signaling in leukemic cells and normal leukocytes and therapeutic implications. J. Leukoc. Biol. 2015, 98, 739–759. [Google Scholar] [CrossRef]
- Marconi, M.; Ascione, B.; Ciarlo, L.; Vona, R.; Garofalo, T.; Sorice, M.; Gianni, A.M.; Locatelli, S.L.; Carlo-Stella, C.; Malorni, W.; et al. Constitutive localization of DR4 in lipid rafts is mandatory for TRAIL-induced apoptosis in B-cell hematologic malignancies. Cell Death Dis. 2013, 4, e863. [Google Scholar] [CrossRef] [Green Version]
- Carbonnelle, D.; Luu, T.H.; Chaillou, C.; Huvelin, J.-M.; Bard, J.-M.; Nazih, H. LXR Activation Down-regulates Lipid Raft Markers FLOT2 and DHHC5 in MCF-7 Breast Cancer Cells. Anticancer. Res. 2017, 37, 4067–4073. [Google Scholar]
- Alawin, O.A.; Ahmed, R.A.; Ibrahim, B.A.; Briski, K.P.; Sylvester, P.W. Antiproliferative effects of γ-tocotrienol are associated with lipid raft disruption in HER2-positive human breast cancer cells. J. Nutr. Biochem. 2016, 27, 266–277. [Google Scholar] [CrossRef]
- Yan, S.; Qu, X.; Xu, L.; Che, X.; Ma, Y.; Zhang, L.; Teng, Y.; Zou, H.; Liu, Y. Bufalin enhances TRAIL-induced apoptosis by redistributing death receptors in lipid rafts in breast cancer cells. Anti-Cancer Drugs 2014, 25, 683–689. [Google Scholar] [CrossRef]
- Gomes, L.; Sorgine, M.; Passos, C.L.A.; Ferreira, C.; De Andrade, I.R.; Silva, J.L.; Atella, G.C.; Mermelstein, C.; Fialho, E. Increase in fatty acids and flotillins upon resveratrol treatment of human breast cancer cells. Sci. Rep. 2019, 9, 13960. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Ko, S.-H.; Shim, J.-S.; Kim, D.-D.; Cho, H.-J. Tumor Targeting and Lipid Rafts Disrupting Hyaluronic Acid-Cyclodextrin-Based Nanoassembled Structure for Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 36628–36640. [Google Scholar] [CrossRef]
- Xu, L.; Hu, X.; Qu, X.; Hou, K.; Zheng, H.; Liu, Y. Cetuximab enhances TRAIL-induced gastric cancer cell apoptosis by promoting DISC formation in lipid rafts. Biochem. Biophys. Res. Commun. 2013, 439, 285–290. [Google Scholar] [CrossRef]
- Xu, L.; Guo, T.; Qu, X.; Hu, X.; Zhang, Y.; Che, X.; Song, H.; Gong, J.; Ma, R.; Li, C.; et al. β-elemene increases the sensitivity of gastric cancer cells to TRAIL by promoting the formation of DISC in lipid rafts. Cell Biol. Int. 2018, 42, 1377–1385. [Google Scholar] [CrossRef]
- Burger, H.-M.; Abel, S.; Gelderblom, W. Modulation of key lipid raft constituents in primary rat hepatocytes by fumonisin B1—Implications for cancer promotion in the liver. Food Chem. Toxicol. 2018, 115, 34–41. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, X.; Wu, L.; Yang, M.; Li, Z.; Gao, Y.; Liu, S.; Zhou, G.; Zhao, J. Lipid rafts promote liver cancer cell proliferation and migration by up-regulation of TLR7 expression. Oncotarget 2016, 7, 63856–63869. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Sharma, N.S.; Kesh, K.; Dauer, P.; Nomura, A.; Giri, B.; Dudeja, V.; Banerjee, S.; Bhattacharya, S.; Saluja, A.; et al. Metastasis and chemoresistance in CD133 expressing pancreatic cancer cells are dependent on their lipid raft integrity. Cancer Lett. 2018, 439, 101–112. [Google Scholar] [CrossRef]
- Chinni, S.R.; Yamamoto, H.; Dong, Z.; Sabbota, A.; Bonfil, R.D.; Cher, M.L. CXCL12/CXCR4 Transactivates HER2 in Lipid Rafts of Prostate Cancer Cells and Promotes Growth of Metastatic Deposits in Bone. Mol. Cancer Res. 2008, 6, 446–457. [Google Scholar] [CrossRef] [Green Version]
- Pirmoradi, L.; Seyfizadeh, N.; Ghavami, S.; Zeki, A.A.; Shojaei, S. Targeting cholesterol metabolism in glioblastoma: A new therapeutic approach in cancer therapy. J. Investig. Med. 2019, 67, 715–719. [Google Scholar] [CrossRef] [Green Version]
- Codini, M.; Cataldi, S.; Ambesi-Impiombato, F.S.; Lazzarini, A.; Floridi, A.; Lazzarini, R.; Curcio, F.; Beccari, T.; Albi, E. Gentamicin Arrests Cancer Cell Growth: The Intriguing Involvement of Nuclear Sphingomyelin Metabolism. Int. J. Mol. Sci. 2015, 16, 2307–2319. [Google Scholar] [CrossRef] [Green Version]
- Codini, M.; Conte, C.; Cataldi, S.; Arcuri, C.; Lazzarini, A.; Ceccarini, M.R.; Patria, F.; Floridi, A.; Mecca, C.; Ambesi-Impiombato, F.S.; et al. Nuclear Lipid Microdomains Regulate Daunorubicin Resistance in Hepatoma Cells. Int. J. Mol. Sci. 2018, 19, 3424. [Google Scholar] [CrossRef] [Green Version]
- Odini, M.; Cataldi, S.; Lazzarini, A.; Tasegian, A.; Ceccarini, M.R.; Floridi, A.; Lazzarini, R.; Ambesi-Impiombato, F.S.; Curcio, F.; Beccari, T.; et al. Why high cholesterol levels help hematological malignancies: Role of nuclear lipid microdomains. Lipids Health Dis. 2016, 15, 4. [Google Scholar]
- Caruso, J.A.; Stemmer, P.M. Proteomic profiling of lipid rafts in a human breast cancer model of tumorigenic progression. Clin. Exp. Metastasis 2011, 28, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Wolczyk, D.; Zaremba-Czogalla, M.; Hryniewicz-Jankowska, A.; Tabola, R.; Grabowski, K.; Sikorski, A.F.; Augoff, K. TNF-α promotes breast cancer cell migration and enhances the concentration of membrane-associated proteases in lipid rafts. Cell. Oncol. 2016, 39, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.E.; Bohin, N.; Boerner, J.L. Src family kinases mediate epidermal growth factor receptor signaling from lipid rafts in breast cancer cells. Cancer Biol. Ther. 2011, 12, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Tan, S.-H.; Ng, S.; Zhou, J.; Yang, N.-D.; Koo, G.-B.; McMahon, K.-A.; Parton, R.G.; Hill, M.M.; Del Pozo, M.A.; et al. Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy 2015, 11, 769–784. [Google Scholar] [CrossRef] [Green Version]
- Alawin, O.A.; Ahmed, R.A.; Dronamraju, V.; Briski, K.P.; Sylvester, P.W. γ-Tocotrienol-induced disruption of lipid rafts in human breast cancer cells is associated with a reduction in exosome heregulin content. J. Nutr. Biochem. 2017, 48, 83–93. [Google Scholar] [CrossRef]
- Li, Y.; Shan, F.; Chen, J. Lipid raft-mediated miR-3908 inhibition of migration of breast cancer cell line MCF-7 by regulating the interactions between AdipoR1 and Flotillin-1. World J. Surg. Oncol. 2017, 15, 69. [Google Scholar] [CrossRef] [Green Version]
- Raghu, H.; Sodadasu, P.K.; Malla, R.; Gondi, C.S.; Estes, N.; Rao, J.S. Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer 2010, 10, 647. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Qin, W.; Chen, Y.; Yuan, B.; Song, X.; Wang, B.; Shen, F.; Fu, J.; Wang, H. Cholesterol inhibits hepatocellular carcinoma invasion and metastasis by promoting CD44 localization in lipid rafts. Cancer Lett. 2018, 429, 66–77. [Google Scholar] [CrossRef]
- Lazzarini, A.; Macchiarulo, A.; Floridi, A.; Coletti, A.; Cataldi, S.; Codini, M.; Lazzarini, R.; Bartoccini, E.; Cascianelli, G.; Ambesi-Impiombato, F.S.; et al. Very Long Chain Fatty Acid Sphingomyelin in Nuclear Lipid Microdomains of Hepatocytes and Hepatoma Cells: Can the Exchange from C24:0 To C16:0 Affect Signal Proteins and Vitamin D Receptor? Mol. Biol. Cell 2015, 26, 2418–2425. [Google Scholar] [CrossRef]
- Gupta, V.K.; Banerjee, S. Isolation of Lipid Raft Proteins from CD133+ Cancer Stem Cells. Methods Mol. Biol. 2017, 1609, 25–31. [Google Scholar]
- Shi, H.; Fang, W.; Liu, M.; Fu, D. Complement component 1, q subcomponent binding protein (C1QBP) in lipid rafts mediates hepatic metastasis of pancreatic cancer by regulating IGF-1/IGF-1R signaling. Int. J. Cancer 2017, 141, 1389–1401. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Pan, Z.; Zhao, M.; Wang, Q.; Qiao, C.; Miao, L.; Ding, X. High cholesterol in lipid rafts reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer. J. Cell. Physiol. 2018, 233, 6722–6732. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, H.; Tan, Y.; Sun, C.; Liang, Y.; Yu, J.; Zou, H. Aggregation of lipid rafts activates c-met and c-Src in non-small cell lung cancer cells. BMC Cancer 2018, 18, 611. [Google Scholar] [CrossRef] [Green Version]
- Ono, Y.J.; Tanabe, A.; Tanaka, T.; Tanaka, Y.; Hayashi, M.; Terai, Y.; Ohmichi, M. Met Signaling Cascade Is Amplified by the Recruitment of Phosphorylated Met to Lipid Rafts via CD24 and Leads to Drug Resistance in Endometrial Cancer Cell Lines. Mol. Cancer Ther. 2015, 14, 2353–2363. [Google Scholar] [CrossRef] [Green Version]
- Hryniewicz-Jankowska, A.; Augoff, K.; Sikorski, A.F. The role of cholesterol and cholesterol-driven membrane raft domains in prostate cancer. Exp. Biol. Med. 2019, 244, 1053–1061. [Google Scholar] [CrossRef]
- Tsuchida, A.; Senda, M.; Ito, A.; Saito, S.; Kiso, M.; Ando, T.; Harduin-Lepers, A.; Matsuda, A.; Furukawa, K.; Furukawa, K. Roles of GalNAc-disialyl Lactotetraosyl Antigens in Renal Cancer Cells. Sci. Rep. 2018, 8, 7017. [Google Scholar] [CrossRef] [Green Version]


| MOLECULES TARGETING LIPID RAFTS IN CANCER | |
|---|---|
| Cell Membrane Lipid Rats | |
| Hydroxypropyl-β-cyclodextrin | [100] |
| Nystatin | [101] |
| TGF-β1 | [100,101] |
| Podoplanin | [102] |
| Caveolin-1 | [103] |
| Flotillin | [104,105] |
| CD44 | [106,107,108] |
| SK3 | [109] |
| Orai1 | [109] |
| TRAIL | [111,117] |
| Edelfosine | [112] |
| Endocannabinoids | [113] |
| Liver X receptor | [118] |
| γ-tocotrienol | [119] |
| Bufalin | [120] |
| Resveratrol | [121] |
| Methyl beta cyclodextrin | [122] |
| Epidermal growth factor receptor | [123] |
| β-elemene | [124] |
| Fumonisin B1 | [125] |
| Antagonist 20S-protopanaxadiol | [126] |
| Lovastatin | [127] |
| CXCL12 | [128] |
| Temozolomide | [129] |
| Nuclear membrane lipid rafts | |
| Gentamicin | [130] |
| Daunorubicin | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codini, M.; Garcia-Gil, M.; Albi, E. Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer. Int. J. Mol. Sci. 2021, 22, 726. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020726
Codini M, Garcia-Gil M, Albi E. Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer. International Journal of Molecular Sciences. 2021; 22(2):726. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020726
Chicago/Turabian StyleCodini, Michela, Mercedes Garcia-Gil, and Elisabetta Albi. 2021. "Cholesterol and Sphingolipid Enriched Lipid Rafts as Therapeutic Targets in Cancer" International Journal of Molecular Sciences 22, no. 2: 726. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020726