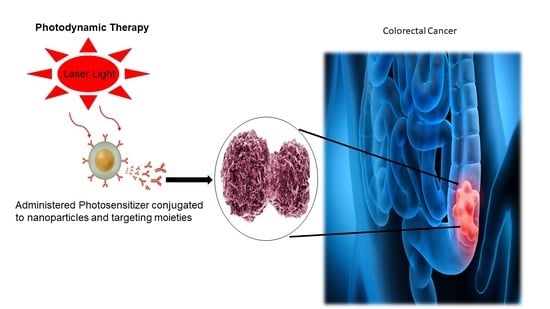
Nanoparticle-Mediated Delivery Systems in Photodynamic Therapy of Colorectal Cancer
Abstract
:1. Introduction
2. PDT in CRC Therapeutics
3. Principle of PDT
4. Cell Death Mechanism Associated with PDT
5. Photosensitizers Used in CRC
5.1. First-Generation PSs
5.2. Second-Generation PSs
5.3. Third-Generation PSs
| PS | Generation | Cell Type | Remarks | Ref. |
|---|---|---|---|---|
| Sinoporphyrin sodium and photofrin | 2nd, 1st | HCT-8 and HCT-116 | The effects of sinoporphyrin sodium-PDT and photofrin-PDT resulted in significant antitumour efficacy | [54] |
| Tetraaryl brominated porphyrin (TBr4) and with the diaryl (BBr2) derivative. | 2nd | Colorectal adenocarcinoma cells, HT29 | Significant reduction in cell growth and necrotic cell death within in vitro and in vivo studies | [55] |
| Gallium (III) phthalocyanine chloride (GaPcCl) | 2nd | Caco-2 | GaPcCl with PDT led to 60% to 80% cell viability cytotoxic and apoptotic cell death. | [56] |
| Tetra 4-(3-(piperidinium-1-ylmethyl) phenoxy substituted zinc (II) phthalocyanine (Zn6a) | 2nd | colorectal carcinoma (HCT-116) | High phototoxicity on HCT-116 cells | [57] |
| Selenium tetrasubstituted zinc (II) phthalocyanines | 2nd | Murine colon carcinoma CT26 | Significant increment in ROS level and efficient antitumour effect. | [58] |
| Hypericin (HY) | 2nd | SW480 and SW620 | HY mediated PDT demonstrated cytotoxic effect and inhibition of tumour cell proliferation in a dose-dependent manner. | [59] |
| Chlorin e6 (Ce6) | 2nd | SW620 | Ce6 mediated PDT significantly reduced the healing and migration rate of colon cells. | [60] |
| 5-aminolevulinic acid | 2nd | SW480 and SW620 | PDT with 5-ALA improved anticancer effects and inhibited of the secretion of cytokines (IL-10) | [61] |
| Ce6 | 2nd | SW480 | Decreased cell survival rate in a dose-dependent manner and significant inhibitory effect on F-actin microfilament and cytoskeleton. | [62] |
| 5-aminolevulinic acid | 2nd | Caco-2 | Cell viability inhibition~62.4%, and improved antitumour efficacy | [63] |
| 5,10,15,20-Tetra(quinolin-2-yl) porphyrin (2TQP) | 2nd | HT29 colorectal adenocarcinoma | 2-TQP displayed effective phototoxic effects with no dark toxicity on cells | [64] |
| Hypericin (HYP) | 2nd | HCT116 and SW620 | Cell proliferation inhibited, and efficient ROS generated by HYP-PDT treatment. Apoptosis was induced | [65] |
| Sinoporphyrin sodium (DVDMS) | 2nd | CX-1 | DVDMS-PDT triggered apoptosis. Inhibitory effect in a dose and time dependent manner | [66] |
6. Current Limitations of CRC PDT
7. Nanotechnology as a Favourable Strategy in PDT for CRC Therapy
7.1. NPs-Mediated PS Delivery in CRC PDT
7.1.1. Passive Targeting Strategy
7.1.2. Active Targeting Strategy
8. PDT Combined with Other Therapies in CRC Treatment
9. Application of 3D Tumour Models in PDT CRC Treatment
10. Clinical Application of PDT in CRC Treatment
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional models |
| 3D | Three-dimensional models |
| AuNPs | Gold nanoparticles |
| CRC | Colorectal cancer |
| ECM | Extracellular matrix |
| e6 | Chlorin e6 |
| EGFR | Epidermal growth factor receptors |
| FOBT | Faecal occult blood test |
| HpD | Hematoporphyrin derivative |
| mAb | Monoclonal antibodies |
| MCTS | Multicellular tumour spheroids |
| NIR | Near infrared |
| NPs | Nanoparticles |
| PDT | Photodynamic therapy |
| PSs | Photosensitizers |
| PTT | Photothermal therapy |
| ROS | Reactive oxygen species |
| TNM | Tumour, nodes, metastasis |
References
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue Invasion and Metastasis: Molecular, Biological and Clinical Perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal Carcinoma: Pathologic Aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Nkune, N.W.; Kruger, C.A.; Abrahamse, H. Possible Enhancement of Photodynamic Therapy (PDT) Colorectal Cancer Treatment When Combined with Cannabidiol. Anticancer. Agents Med. Chem. 2021, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.; Rutter, M.D. Colorectal Cancer Screening—Who, How, and When? Clin. Endosc. 2018, 51, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Simelane, N.W.N.; Kruger, C.A.; Abrahamse, H. Photodynamic Diagnosis and Photodynamic Therapy of Colorectal Cancer in Vitro and in Vivo. RSC Adv. 2020, 10, 41560–41576. [Google Scholar] [CrossRef]
- Mishra, J.; Dromund, J.; Quazi, S.H.; Karanki, S.S.; Shaw, J.; Chen, B.; Kumar, N. Prospective of Colon Cancer Treatments and Scope for Combinatorial Approach to Enhanced Cancer Cell Apoptosis. Crit. Rev. Oncol. Hematol. 2013, 86, 232–250. [Google Scholar] [CrossRef] [Green Version]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and Effective Photodynamic Therapy for Cancer Using Functionalized Nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment-an Update Review. J. Cancer Metastasis Treat. 2019, 5. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Benov, L. Photodynamic Therapy: Current Status and Future Directions. Med. Princ. Pr. 2014, 24, 14–28. [Google Scholar] [CrossRef]
- Hodgkinson, N.; Kruger, C.A.; Abrahamse, H. Targeted Photodynamic Therapy as Potential Treatment Modality for the Eradication of Colon Cancer and Colon Cancer Stem Cells. Tumour. Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Kruger, C.; Abrahamse, H. Utilisation of Targeted Nanoparticle Photosensitiser Drug Delivery Systems for the Enhancement of Photodynamic Therapy. Molecules 2018, 23, 2628. [Google Scholar] [CrossRef] [Green Version]
- Kawczyk-Krupka, A.; Bugaj, A.M.; Latos, W.; Zaremba, K.; Wawrzyniec, K.; Kucharzewski, M.; Sieroń, A. Photodynamic Therapy in Colorectal Cancer Treatment—The State of the Art in Preclinical Research. Photodiagn. Photodyn. Ther. 2016, 13, 158–174. [Google Scholar] [CrossRef]
- Mohammad-Hadi, L.; MacRobert, A.J.; Loizidou, M.; Yaghini, E. Photodynamic Therapy in 3D Cancer Models and the Utilisation of Nanodelivery Systems. Nanoscale 2018, 10, 1570–1581. [Google Scholar] [CrossRef] [Green Version]
- Evans, C.L. Three-Dimensional in Vitro Cancer Spheroid Models for Photodynamic Therapy: Strengths and Opportunities. Front. Phys. 2015, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Kruger, C.A.; Abrahamse, H. Targeted Photodynamic Therapy as Potential Treatment Modality for the Eradication of Colon Cancer; IntechOpen: London, UK, 2019; ISBN 978-1-78984-400-9. [Google Scholar]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Shirasu, N.; Nam, S.O.; Kuroki, M. Tumor-Targeted Photodynamic Therapy. Anticancer Res. 2013, 33, 2823–2831. [Google Scholar]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Mesquita, M.Q.; Dias, C.J.; Gamelas, S.; Fardilha, M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Mesquita, M.Q.; Dias, C.J.; Gamelas, S.; Fardilha, M.; et al. An Insight on the Role of Photosensitizer Nanocarriers for Photodynamic Therapy. An. Acad. Bras. Ciências 2018, 90, 1101–1130. [Google Scholar] [CrossRef] [Green Version]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- De Silva, P.; Saad, M.A.; Thomsen, H.C.; Bano, S.; Ashraf, S.; Hasan, T. Photodynamic Therapy, Priming and Optical Imaging: Potential Co-Conspirators in Treatment Design and Optimization—A Thomas Dougherty Award for Excellence in PDT Paper. J. Porphyr. Phthalocyanines 2020, 24, 1320–1360. [Google Scholar] [CrossRef]
- Yan, J.; Wang, C.; Jiang, X.; Wei, Y.; Wang, Q.; Cui, K.; Xu, X.; Wang, F.; Zhang, L. Application of Phototherapeutic-Based Nanoparticles in Colorectal Cancer. Int. J. Biol. Sci. 2021, 17, 1361–1381. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Two—Cellular Signaling, Cell Metabolism and Modes of Cell Death. Photodiagn. Photodyn. 2005, 2, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Panzarini, E.; Inguscio, V.; Dini, L. Overview of Cell Death Mechanisms Induced by Rose Bengal Acetate-Photodynamic Therapy. Int. J. Photoenergy 2011, 2011, e713726. [Google Scholar] [CrossRef]
- Kessel, D.; Oleinick, N.L. Cell Death Pathways Associated with Photodynamic Therapy: An Update. Photochem. Photobiol. 2018, 94, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Mahalingam, S.M.; Ordaz, J.D.; Low, P.S. Targeting of a Photosensitizer to the Mitochondrion Enhances the Potency of Photodynamic Therapy. ACS Omega 2018, 3, 6066–6074. [Google Scholar] [CrossRef]
- Abrahamse, H.; Houreld, N.N. Genetic Aberrations Associated with Photodynamic Therapy in Colorectal Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekhejane, P.R.; Houreld, N.N.; Abrahamse, H. Multiorganelle Localization of Metallated Phthalocyanine Photosensitizer in Colorectal Cancer Cells (DLD-1 and CaCo-2) Enhances Efficacy of Photodynamic Therapy. Int. J. Photoenergy 2014, 2014, e383027. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Xu, W.; Wu, H.; Wang, X.; Gong, Q.; Liu, C.; Liu, J.; Zhou, L. Photodynamic Therapy Induces Autophagy-Mediated Cell Death in Human Colorectal Cancer Cells via Activation of the ROS/JNK Signaling Pathway. Cell Death Dis. 2020, 11. [Google Scholar] [CrossRef]
- Wei, M.-F.; Chen, M.-W.; Chen, K.-C.; Lou, P.-J.; Lin, S.Y.-F.; Hung, S.-C.; Hsiao, M.; Yao, C.-J.; Shieh, M.-J. Autophagy Promotes Resistance to Photodynamic Therapy-Induced Apoptosis Selectively in Colorectal Cancer Stem-like Cells. Autophagy 2014, 10, 1179–1192. [Google Scholar] [CrossRef] [Green Version]
- Tsubone, T.M.; Martins, W.K.; Pavani, C.; Junqueira, H.C.; Itri, R.; Baptista, M.S. Enhanced Efficiency of Cell Death by Lysosome-Specific Photodamage. Sci. Rep. 2017, 7, 6734. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Hu, Y.; Wang, H. Targeting Antitumor Immune Response for Enhancing the Efficacy of Photodynamic Therapy of Cancer: Recent Advances and Future Perspectives. Oxidative Med. Cell. Longev. 2016, 2016, e5274084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, S.; Chan, T.A.; Hasan, T.; Maytin, E.V. Current Prospects for Treatment of Solid Tumors via Photodynamic, Photothermal, or Ionizing Radiation Therapies Combined with Immune Checkpoint Inhibition (A Review). Pharmaceuticals 2021, 14, 447. [Google Scholar] [CrossRef]
- Beltrán Hernández, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic Photodynamic Therapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janas, K.; Boniewska-Bernacka, E.; Dyrda, G.; Słota, R. Porphyrin and Phthalocyanine Photosensitizers Designed for Targeted Photodynamic Therapy of Colorectal Cancer. Bioorg. Med. Chem. 2021, 30, 115926. [Google Scholar] [CrossRef]
- Sun, B.; Li, W.; Liu, N. Curative Effect of the Recent Photofrin Photodynamic Adjuvant Treatment on Young Patients with Advanced Colorectal Cancer. Oncol. Lett. 2016, 11, 2071–2074. [Google Scholar] [CrossRef] [Green Version]
- Kawczyk-Krupka, A.; Bugaj, A.; Latos, W.; Zaremba, K.; Wawrzyniec, K.; Sieron, A. Photodynamic Therapy in Colorectal Cancer Treatment: The State of the Art in Clinical Trials. Photodiagn. Photodyn. Ther. 2015, 12, 545–553. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy–Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Weijer, R.; Broekgaarden, M.; Kos, M.; van Vught, R.; Rauws, E.A.J.; Breukink, E.; van Gulik, T.M.; Storm, G.; Heger, M. Enhancing Photodynamic Therapy of Refractory Solid Cancers: Combining Second-Generation Photosensitizers with Multi-Targeted Liposomal Delivery. J. Photochem. Photobiol. C Photochem. Rev. 2015, 23, 103–131. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Li, Z.; Chen, H.; Gao, Y. Nanoparticle-Based Drug Delivery Systems for Controllable Photodynamic Cancer Therapy. Eur. J. Pharm. Sci. 2020, 144, 105213. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, Y.; Liu, N.; Suo, Y. Nanoparticles Loading Porphyrin Sensitizers in Improvement of Photodynamic Therapy for Ovarian Cancer. Photodiagn. Photodyn. Ther. 2021, 33, 102156. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, A.; Bhupathiraju, N.V.S.D.K.; Arianna, G.; Tiwari, K.; Drain, C.M. Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics. Chem. Rev. 2015, 115, 10261–10306. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, H.; Amano, T.; Qin, H.; Zheng, L.; Takahashi, A.; Zhao, S.; Tooyama, I.; Murakami, T.; Komatsu, N. Efficient Delivery of Chlorin E6 into Ovarian Cancer Cells with Octalysine Conjugated Superparamagnetic Iron Oxide Nanoparticles for Effective Photodynamic Therapy. J. Mater. Chem. B 2016, 4, 7741–7748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yu, Y.; Kang, L.; Lu, Y. Effects of Chlorin E6-Mediated Photodynamic Therapy on Human Colon Cancer SW480 Cells. Int. J. Clin. Exp. Med. 2014, 7, 4867–4876. [Google Scholar]
- Demazeau, M.; Gibot, L.; Mingotaud, A.-F.; Vicendo, P.; Roux, C.; Lonetti, B. Rational Design of Block Copolymer Self-Assemblies in Photodynamic Therapy. Beilstein. J. Nanotechnol. 2020, 11, 180–212. [Google Scholar] [CrossRef]
- Li, X.-Y.; Tan, L.-C.; Dong, L.-W.; Zhang, W.-Q.; Shen, X.-X.; Lu, X.; Zheng, H.; Lu, Y.-G. Susceptibility and Resistance Mechanisms During Photodynamic Therapy of Melanoma. Front. Oncol. 2020, 10, 597. [Google Scholar] [CrossRef]
- Shi, R.; Li, C.; Jiang, Z.; Li, W.; Wang, A.; Wei, J. Preclinical Study of Antineoplastic Sinoporphyrin Sodium-PDT via In Vitro and In Vivo Models. Molecules 2017, 22, 112. [Google Scholar] [CrossRef]
- Laranjo, M.; Serra, A.C.; Abrantes, M.; Piñeiro, M.; Gonçalves, A.C.; Casalta-Lopes, J.; Carvalho, L.; Sarmento-Ribeiro, A.B.; Rocha-Gonsalves, A.; Botelho, F. 2-Bromo-5-Hydroxyphenylporphyrins for Photodynamic Therapy: Photosensitization Efficiency, Subcellular Localization and in Vivo Studies. Photodiagn. Photodyn. 2013, 10, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Maduray, K.; Odhav, B. Efficacy of Gallium Phthalocyanine as a Photosensitizing Agent in Photodynamic Therapy for the Treatment of Cancer. In Proceedings of the Optics in Health Care and Biomedical Optics V, Beijing, China, 11 December 2012; International Society for Optics and Photonics, Photonics Asia: Beijing, China, 2012; Volume 8553, p. 85530G. [Google Scholar]
- Barut, B.; Yalçın, C.Ö.; Demirbaş, Ü.; Özel, A. Photochemical and in Vitro Phototoxic Properties of Zn (II) Phthalocyanine Bearing Piperidinium Groups on Different Cell Lines. J. Organomet. Chem. 2020, 921, 121358. [Google Scholar] [CrossRef]
- Ezquerra Riega, S.D.; Chiarante, N.; Valli, F.; Marino, J.; Roguin, L.P.; Awruch, J.; García Vior, M.C. Novel Hydro- and Lipo-Philic Selenium Zinc(II) Phthalocyanines: Synthesis, Photophysical Properties and Photodynamic Effects on CT26 Colon Carcinoma Cells. Dye. Pigment. 2018, 156, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Kaleta-Richter, M.; Aebisher, D.; Jaworska, D.; Czuba, Z.; Cieślar, G.; Kawczyk-Krupka, A. The Influence of Hypericin-Mediated Photodynamic Therapy on Interleukin-8 and -10 Secretion in Colon Cancer Cells. Integr. Cancer 2020, 19, 1534735420918931. [Google Scholar] [CrossRef]
- Wufuer, R.; Ma, H.-X.; Luo, M.-Y.; Xu, K.-Y.; Kang, L. Downregulation of Rac1/PAK1/LIMK1/Cofilin Signaling Pathway in Colon Cancer SW620 Cells Treated with Chlorin E6 Photodynamic Therapy. Photodiagn. Photodyn. Ther. 2021, 33, 102143. [Google Scholar] [CrossRef]
- Kawczyk-Krupka, A.; Czuba, Z.; Latos, W.; Wasilewska, K.; Verwanger, T.; Krammer, B.; Sieroń, A. Influence of ALA-Mediated Photodynamic Therapy on Secretion of Interleukins 6, 8 and 10 by Colon Cancer Cells in Vitro. Photodiagn. Photodyn. Ther. 2018, 22, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yang, K.; Li, H.; Luo, M.; Wufuer, R.; Kang, L. Photodynamic Effect of Chlorin E6 on Cytoskeleton Protein of Human Colon Cancer SW480 Cells. Photodiagn. Photodyn. Ther. 2021, 33, 102201. [Google Scholar] [CrossRef]
- Şueki, F.; Ruhi, M.K.; Gülsoy, M. The Effect of Curcumin in Antitumor Photodynamic Therapy: In Vitro Experiments with Caco-2 and PC-3 Cancer Lines. Photodiagn. Photodyn. Ther. 2019, 27, 95–99. [Google Scholar] [CrossRef]
- Costa, L.D.; Silva, J.d.A.e.; Fonseca, S.M.; Arranja, C.T.; Urbano, A.M.; Sobral, A.J.F.N. Photophysical Characterization and in Vitro Phototoxicity Evaluation of 5,10,15,20-Tetra(Quinolin-2-Yl)Porphyrin as a Potential Sensitizer for Photodynamic Therapy. Molecules 2016, 21, 439. [Google Scholar] [CrossRef] [Green Version]
- Hypericin-Mediated Photodynamic Therapy Inhibits Growth of Colorectal Cancer Cells via Inducing S Phase Cell Cycle Arrest and Apoptosis. Eur. J. Pharmacol. 2021, 900, 174071. [CrossRef]
- Kong, F.; Zou, H.; Liu, X.; He, J.; Zheng, Y.; Xiong, L.; Miao, X. MiR-7112-3p Targets PERK to Regulate the Endoplasmic Reticulum Stress Pathway and Apoptosis Induced by Photodynamic Therapy in Colorectal Cancer CX-1 Cells. Photodiagn. Photodyn. Ther. 2020, 29, 101663. [Google Scholar] [CrossRef] [PubMed]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting Immunogenic Cancer Cell Death by Photodynamic Therapy: Past, Present and Future. J. Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug Delivery Systems for Phthalocyanines for Photodynamic Therapy. Anticancer Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef] [Green Version]
- Chizenga, E.P.; Abrahamse, H. Nanotechnology in Modern Photodynamic Therapy of Cancer: A Review of Cellular Resistance Patterns Affecting the Therapeutic Response. Pharmaceutics 2020, 12, 632. [Google Scholar] [CrossRef]
- dos Santos, A.F.; Arini, G.S.; de Almeida, D.R.Q.; Labriola, L. Nanophotosensitizers for Cancer Therapy: A Promising Technology? J. Phys. Mater. 2021, 4, 032006. [Google Scholar] [CrossRef]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.E. The Impact of Nanoparticles on the Immune System: A Gray Zone of Nanomedicine. J. Immunol. Sci. 2021, 5. [Google Scholar] [CrossRef]
- Montaseri, H.; Kruger, C.; Abrahamse, H. Inorganic Nanoparticles Applied for Active Targeted Photodynamic Therapy of Breast Cancer. Pharmaceutics 2021, 13, 296. [Google Scholar] [CrossRef]
- Sadasivam, M.; Avci, P.; Gupta, G.K.; Lakshmanan, S.; Chandran, R.; Huang, Y.-Y.; Kumar, R.; Hamblin, M.R. Self-Assembled Liposomal Nanoparticles in Photodynamic Therapy. Eur. J. Nanomed. 2013, 5, 115–129. [Google Scholar] [CrossRef]
- Bakhshizadeh, M.; Moshirian, T.; Esmaily, H.; Rajabi, O.; Nassirli, H.; Sazgarnia, A. Sonophotodynamic Therapy Mediated by Liposomal Zinc Phthalocyanine in a Colon Carcinoma Tumor Model: Role of Irradiating Arrangement. Iran. J. Basic Med. Sci. 2017, 20, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.W.K.; Chu, E.S.M.; Huang, Z.; Olivo, M.C.; Ip, D.C.W.; Yow, C.M.N. An in Vitro Investigation of Photodynamic Efficacy of FosPeg® on Human Colon Cancer Cells. J. Innov. Opt. Health Sci. 2015, 8, 1550027. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y.; et al. Multi-Functional Liposome: A Powerful Theranostic Nano-Platform Enhancing Photodynamic Therapy. Adv. Sci. 2021, 2100876. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Zhang, L.; Wang, J.; He, Y.; Xin, J.; Wang, S.; Xu, H.; Zhang, Z. Gold Nanoparticle Mediated Phototherapy for Cancer. J. Nanomater. 2016, 2016, e5497136. [Google Scholar] [CrossRef] [Green Version]
- Naidoo, C.; Kruger, C.A.; Abrahamse, H. Photodynamic Therapy for Metastatic Melanoma Treatment: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818791795. [Google Scholar] [CrossRef] [Green Version]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer Targeting with Biomolecules: A Comparative Study of Photodynamic Therapy Efficacy Using Antibody or Lectin Conjugated Phthalocyanine-PEG Gold Nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Yurt, F.; Sarı, F.A.; Ince, M.; Colak, S.G.; Er, O.; Soylu, H.M.; Kurt, C.C.; Avci, C.B.; Gunduz, C.; Ocakoglu, K. Photodynamic Therapy and Nuclear Imaging Activities of SubPhthalocyanine Integrated TiO2 Nanoparticles. J. Photochem. Photobiol. A Chem. 2018, 367, 45–55. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Kimura, E.; Rubira, A.F.; Muniz, E.C. Curcumin and Silver Nanoparticles Carried out from Polysaccharide-Based Hydrogels Improved the Photodynamic Properties of Curcumin through Metal-Enhanced Singlet Oxygen Effect. Mater. Sci. Eng. C 2020, 112, 110853. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, M.; Caruso, E.; Guerrini, A.; Ferroni, C.; Banfi, S.; Gariboldi, M.; Monti, E.; Sotgiu, G.; Varchi, G. Core-Shell Poly-Methyl Methacrylate Nanoparticles Covalently Functionalized with a Non-Symmetric Porphyrin for Anticancer Photodynamic Therapy. J. Photochem. Photobiol. B 2018, 186, 169–177. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer Nanotechnology: The Impact of Passive and Active Targeting in the Era of Modern Cancer Biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.H.; Jeong, Y.-I.; Kim, H.Y.; Son, G.M.; Lee, H.L.; Chung, C.-W.; Chu, C.W.; Kang, D.H. Enhanced Photosensing and Photodynamic Treatment of Colon Cancer Cells Using Methoxy Poly(Ethylene Glycol)-Conjugated Chlorin E6. J. Nanosci. Nanotechnol. 2018, 18, 1131–1136. [Google Scholar] [CrossRef]
- Bretin, L.; Pinon, A.; Bouramtane, S.; Ouk, C.; Richard, L.; Perrin, M.-L.; Chaunavel, A.; Carrion, C.; Bregier, F.; Sol, V.; et al. Photodynamic Therapy Activity of New Porphyrin-Xylan-Coated Silica Nanoparticles in Human Colorectal Cancer. Cancers 2019, 11, 1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Zhao, Y.; Xu, Y.; Zhu, C.; Liu, T.; Wang, K. Chitosan Nanoparticles for Oral Photothermally Enhanced Photodynamic Therapy of Colon Cancer. Int. J. Pharm. 2020, 589, 119763. [Google Scholar] [CrossRef]
- Viard, M.; Reichard, H.; Shapiro, B.A.; Durrani, F.A.; Marko, A.J.; Watson, R.M.; Pandey, R.K.; Puri, A. Design and Biological Activity of Novel Stealth Polymeric Lipid Nanoparticles for Enhanced Delivery of Hydrophobic Photodynamic Therapy Drugs. Nanomedicine 2018, 14, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.; Min, H.S.; Li, L.; Tran, T.H.; Lee, Y.; Kwon, I.C.; Choi, K.; Kim, K.; Huh, K.M. Cancer Cell-Specific Photoactivity of Pheophorbide a-Glycol Chitosan Nanoparticles for Photodynamic Therapy in Tumor-Bearing Mice. Biomaterials 2013, 34, 6454–6463. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, W.; Kim, G.; Hah, H.J.; Lee, Y.-E.K.; Kopelman, R.; Ethirajan, M.; Gupta, A.; Goswami, L.N.; Pera, P.; et al. Novel Methods to Incorporate Photosensitizers Into Nanocarriers for Cancer Treatment by Photodynamic Therapy. Lasers Surg. Med. 2011, 43, 686–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.-L.; Lai, P.-S.; Lin, F.-H.; Yueh-Hsiu Wu, S.; Shieh, M.-J. Dual Chemotherapy and Photodynamic Therapy in an HT-29 Human Colon Cancer Xenograft Model Using SN-38-Loaded Chlorin-Core Star Block Copolymer Micelles. Biomaterials 2009, 30, 3614–3625. [Google Scholar] [CrossRef] [PubMed]
- Sardoiwala, M.N.; Kushwaha, A.C.; Dev, A.; Shrimali, N.; Guchhait, P.; Karmakar, S.; Choudhury, S.R. Hypericin-Loaded Transferrin Nanoparticles Induce PP2A-Regulated BMI1 Degradation in Colorectal Cancer-Specific Chemo-Photodynamic Therapy. ACS Biomater. Sci. Eng. 2020, 6, 3139–3153. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Wang, J.; Yuan, A.; Sun, M.; Wu, J.; Hu, Y. Self-Assembled IR780-Loaded Transferrin Nanoparticles as an Imaging, Targeting and PDT/PTT Agent for Cancer Therapy. Sci. Rep. 2016, 6, 27421. [Google Scholar] [CrossRef] [PubMed]
- Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Epidermal Growth Factor Receptor Conjugated Fucoidan/Alginates Loaded Hydrogel for Activating EGFR/AKT Signaling Pathways in Colon Cancer Cells during Targeted Photodynamic Therapy. Int. J. Biol. Macromol. 2020, 158, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhong, H.; Liang, R.; Lan, T.; Zhu, X.; Huang, S.; Wang, Y.; Shao, J.; Shuai, X.; Wei, B. Multifunctional Nanodrug Mediates Synergistic Photodynamic Therapy and MDSCs-Targeting Immunotherapy of Colon Cancer. Adv. Sci. 2021, 2100712. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Kataoka, H.; Yano, S.; Tanaka, M.; Moriwaki, K.; Akashi, H.; Suzuki, S.; Mori, Y.; Kubota, E.; Tanida, S.; et al. A Novel Photodynamic Therapy Targeting Cancer Cells and Tumor-Associated Macrophages. Mol. Cancer 2015, 14, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Wang, H.; Zhang, Z. A Valproic Acid-Modified Platinum Diimine Complex as Potential Photosensitizer for Photodynamic Therapy. J. Inorg. Biochem. 2021, 222, 111508. [Google Scholar] [CrossRef]
- Ruttala, H.B.; Ramasamy, T.; Ruttala, R.R.T.; Tran, T.H.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Mitochondria-Targeting Multi-Metallic ZnCuO Nanoparticles and IR780 for Efficient Photodynamic and Photothermal Cancer Treatments. J. Mater. Sci. Technol. 2021, 86, 139–150. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, D.; Chen, Z.; Chen, J. Photodynamic Therapy of Drug-Resistant Human Colon Adenocarcinoma Using Verteporfin-Loaded TPGS Nanoparticles with Tumor Homing and Penetrating Peptide Functionalization. RSC Adv. 2016, 6, 100984–100992. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal Cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Fan, G.; Wu, H.; Liu, C.; Zhan, Y.; Qiu, Y.; Shou, C.; Gao, F.; Zhang, J.; Yin, P.; et al. Photodynamic Therapy Synergize with PD-L1 Checkpoint Blockade for Immunotherapy of Colorectal Cancer by Multifunctional Nanoparticle. Mol. Ther. 2021. [Google Scholar] [CrossRef]
- He, C.; Duan, X.; Guo, N.; Chan, C.; Poon, C.; Weichselbaum, R.R.; Lin, W. Core-Shell Nanoscale Coordination Polymers Combine Chemotherapy and Photodynamic Therapy to Potentiate Checkpoint Blockade Cancer Immunotherapy. Nat. Commun. 2016, 7, 12499. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xu, L.; Wang, C.; Yang, R.; Zhuang, Q.; Han, X.; Dong, Z.; Zhu, W.; Peng, R.; Liu, Z. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano 2017, 12, 4463–4474. [Google Scholar] [CrossRef]
- Seo, S.-H.; Kim, B.-M.; Joe, A.; Han, H.-W.; Chen, X.; Cheng, Z.; Jang, E.-S. NIR-Light-Induced Surface-Enhanced Raman Scattering for Detection and Photothermal/Photodynamic Therapy of Cancer Cells Using Methylene Blue-Embedded Gold Nanorod@SiO2 Nanocomposites. Biomaterials 2014, 35, 3309–3318. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ouyang, X.; Chen, J.; Hu, Y.; Sun, X.; Yu, Z. Nanoparticulate Photosensitizer Decorated with Hyaluronic Acid for Photodynamic/Photothermal Cancer Targeting Therapy. Nanomedicine 2019, 14, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broekgaarden, M.; Rizvi, I.; Bulin, A.-L.; Petrovic, L.; Goldschmidt, R.; Massodi, I.; Celli, J.P.; Hasan, T. Neoadjuvant Photodynamic Therapy Augments Immediate and Prolonged Oxaliplatin Efficacy in Metastatic Pancreatic Cancer Organoids. Oncotarget 2018, 9, 13009–13022. [Google Scholar] [CrossRef] [PubMed]
- Khot, M.I.; Perry, S.L.; Maisey, T.; Armstrong, G.; Andrew, H.; Hughes, T.A.; Kapur, N.; Jayne, D.G. Inhibiting ABCG2 Could Potentially Enhance the Efficacy of Hypericin-Mediated Photodynamic Therapy in Spheroidal Cell Models of Colorectal Cancer. Photodiagn. Photodyn. Ther. 2018, 23, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamberti, M.J.; Pansa, M.F.; Vera, R.E.; Vittar, N.B.R.; Rivarola, V.A. Photodynamic Therapy Potentiates the Paracrine Endothelial Stimulation by Colorectal Cancer. Laser Phys. 2014, 24, 115602. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Pereira, P.M.R.; Berisha, N.; Bhupathiraju, N.V.S.D.K.; Fernandes, R.; Tomé, J.P.C.; Drain, C.M. Cancer Cell Spheroids Are a Better Screen for the Photodynamic Efficiency of Glycosylated Photosensitizers. PLoS ONE 2017, 12, e0177737. [Google Scholar] [CrossRef]
- Till, U.; Gibot, L.; Vicendo, P.; Rols, M.-P.; Gaucher, M.; Violleau, F.; Mingotaud, A.-F. Crosslinked Polymeric Self-Assemblies as an Efficient Strategy for Photodynamic Therapy on a 3D Cell Culture. RSC Adv. 2016, 6, 69984–69998. [Google Scholar] [CrossRef] [Green Version]
- Gibot, L.; Lemelle, A.; Till, U.; Moukarzel, B.; Mingotaud, A.-F.; Pimienta, V.; Saint-Aguet, P.; Rols, M.-P.; Gaucher, M.; Violleau, F.; et al. Polymeric Micelles Encapsulating Photosensitizer: Structure/Photodynamic Therapy Efficiency Relation. Biomacromolecules 2014, 15, 1443–1455. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Jeong, M.; Lee, H.; Goh, U.; Kim, H.; Kim, B.; Park, J.-H. Liposome-Based Engineering of Cells to Package Hydrophobic Compounds in Membrane Vesicles for Tumor Penetration. Nano Lett. 2015, 15, 2938–2944. [Google Scholar] [CrossRef] [PubMed]
- Welbourn, H.; Duthie, G.; Powell, J.; Moghissi, K. Can Photodynamic Therapy Be the Preferred Treatment Option for Anal Intraepithelial Neoplasia? Initial Results of a Pilot Study. Photodiagn. Photodyn. Ther. 2014, 11, 20–21. [Google Scholar] [CrossRef]
- Zou, H.; Wang, F.; Zhou, J.-J.; Liu, X.; He, Q.; Wang, C.; Zheng, Y.-W.; Wen, Y.; Xiong, L. Application of Photodynamic Therapy for Liver Malignancies. J. Gastrointest. Oncol. 2020, 11, 431–442. [Google Scholar] [CrossRef]
- Vogl, T.J.; Eichler, K.; Mack, M.G.; Zangos, S.; Herzog, C.; Thalhammer, A.; Engelmann, K. Interstitial Photodynamic Laser Therapy in Interventional Oncology. Eur. Radiol. 2004, 14, 1063–1073. [Google Scholar] [CrossRef]



| In Vivo CRC PDT Studies Reported on Passive Nanoparticle-Based Photosensitizers | ||||
|---|---|---|---|---|
| Nanosystem | PS | Key Findings | Cell Type | Ref. |
| chitosan nanoparticles (CS NPs) | Encaspulated 5-Aminolevulinic acid 5-ALA and photothermal (IR780) | Superior photodynamic cytotoxicity effects, higher tumour accumulation | mouse colon tumours CT-26 cells | [87] |
| lipid nanoparticles | HPPH | Effective accumulated in colon tumours and enhanced anticancer activity | Murine CT-26 colon carcinoma and HT29 tumour bearing mice | [88] |
| PheoA-ss-GC nanoparticles (PheoA-ss-CNPs), | pheophorbidea (PheoA) | Increased selective accumulation and significant reduction in tumour growth | HT-29 tumour-bearing mice | [89] |
| Functionalized polyacrylamide (AFPAA) | 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a (HPPH) | Improved localisation and the tumour response to the treatment was approximately 40%. | BALB/c mice bearing Colon26 tumours | [90] |
| Chlorin-core star-shaped block copolymer (CSBC) | The combinative effects of chemotherapy and PDT -SN-38/CBSC demonstrated significant anticancer efficacy. | HT-29 xenograft model. | [91] | |
| In Vitro CRC PDT Studies Reported on Active Nanoparticle-Based Photosensitizers | |||||
|---|---|---|---|---|---|
| Nanosystem | Ligand/Moieties | PS | Key Findings | Cell Type | Ref. |
| EGFR-hydrogel | EGFR antibody | chlorin e6 (Ce6) | Excellent synergistic anticancer effect with increased protein expression levels. | HT-29 (Human colon cancer cell lines) | [95] |
| Liposomes encapsulated Ce6 and phosphoinositide 3-kinase gamma (PI3Kγ) inhibitor IPI-549 | IPI-549 | chlorin e6 | Efficient tumour targeting, and anticancer activity | CT26 cells | [96] |
| Mannose-conjugated chlorin (M-chlorin) | Mannose- | M-chlorin | Higher tumour selectivity, increased cytotoxicity, and significantly suppressed tumour growth | HT29, HCT116, CT26 cells | [97] |
| VPA moiety-platinum diimine complexes | VPA moiety | Platinum diimine complexes | Minimal dark toxicity and improved cytotoxic effect on cancer cells | SW480 human colon cancer cell line | [98] |
| TPP-conjugated polymer-lipid hybrid nanoparticles | Triphenylphosphonium (TPP) | ZCNP/IR780 | Enhanced specific mitochondria-targeting and enhanced anticancer effect. | Human colon carcinoma (HT-29) and HT-29 cell-bearing xenograft | [99] |
| In vivo CRC PDT studies reported on active nanoparticle-based photosensitizers | |||||
| Nanosystem | PS | Key Findings | Cell Type | Ref. | |
| Liposome encapsulated photosensitizer chlorin e6 (Ce6) and phosphoinositide 3-kinase gamma (PI3Kγ) inhibitor IPI-549 | IPI-549 | Ce6 | The nanoformulations improved PDT therapeutic effect | CT26 cells | [96] |
| Verteporfin-loaded D-α-tocopheryl polyethylene glycol succinate (TPGS) nanoparticles modified with tLyp-1 tumour homing and peptide tLyp-1 decoration (t-NP) | tLyp-1 decoration (t-NP) peptide | Verteporfin (VP) | Higher tumour selectivity of PS, inhibition of tumour growth and enhanced in vivo photodynamic effects. | HCT15 colon cells | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winifred Nompumelelo Simelane, N.; Abrahamse, H. Nanoparticle-Mediated Delivery Systems in Photodynamic Therapy of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 12405. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222212405
Winifred Nompumelelo Simelane N, Abrahamse H. Nanoparticle-Mediated Delivery Systems in Photodynamic Therapy of Colorectal Cancer. International Journal of Molecular Sciences. 2021; 22(22):12405. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222212405
Chicago/Turabian StyleWinifred Nompumelelo Simelane, Nokuphila, and Heidi Abrahamse. 2021. "Nanoparticle-Mediated Delivery Systems in Photodynamic Therapy of Colorectal Cancer" International Journal of Molecular Sciences 22, no. 22: 12405. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222212405