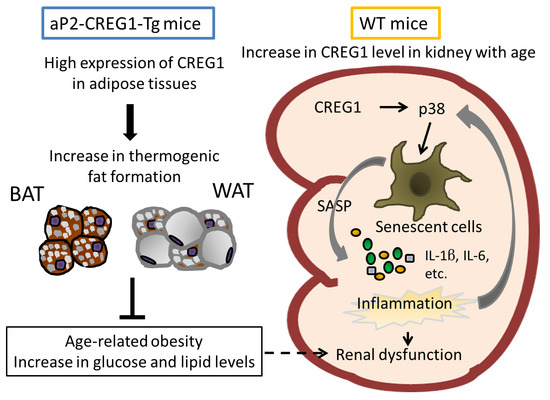
Effects of CREG1 on Age-Associated Metabolic Phenotypes and Renal Senescence in Mice
Abstract
:1. Introduction
2. Results
2.1. Improvement of Age-Related Obesity in aP2-CREG1-Tg Mice
2.2. Effect of Age on CREG1 Expression in Various Tissues of aP2-CREG1-Tg Mice
2.3. Stimulation of Browning and Improvement of Kidney Pathophysiology in Aged aP2-CREG1-Tg Mice
2.4. Inhibition of Renal Senescence in Aged aP2-CREG1-Tg Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Blood Glucose Level Measurement
4.3. Lipid Measurement
4.4. Gene-Expression Analysis
4.5. Protein Analysis
4.6. Histologic Analysis
4.7. Evaluation of Renal Function
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aP2 | Adipocyte P2 |
| C57BL/6J | C57 black 6 Jackson |
| CIDEA | Cell death-inducing DFFA-like effector A |
| CREG1 | Cellular repressor of adenovirus early region 1A-stimulated genes 1 |
| DIO | Diet-induced obesity |
| FGF21 | Fibroblast growth factor 21 |
| GM | Gastrocnemius muscle |
| GWAT | Gonadal white adipose tissue |
| H&E | Hematoxylin and eosin |
| iBAT | Interscapular brown adipose tissue |
| IWAT | Inguinal white adipose tissue |
| PAS | Periodic acid–Schiff |
| pRb | Retinoblastoma tumor suppressor protein |
| PRDM16 | PR domain containing 16 |
| RWAT | Retroperitoneal white adipose tissue |
| SASP | Senescence-associated secretory phenotype |
| Tg | Transgenic |
| UCP1 | Uncoupling protein 1 |
| WT | Wild type |
References
- Veal, E.A.; Eisenstein, M.; Tseng, Z.H.; Gill, G. A Cellular Repressor of E1A-Stimulated Genes That Inhibits Activation by E2F. Mol. Cell. Biol. 1998, 18, 5032–5041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veal, E.; Groisman, R.; Eisenstein, M.; Gill, G. The secreted glycoprotein CREG enhances differentiation of NTERA-2 human embryonal carcinoma cells. Oncogene 2000, 19, 2120–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moolmuang, B.; Tainsky, M.A. CREG1 enhances p16INK4a-induced cellular senescence. Cell Cycle 2011, 10, 518–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, Z.; Cai, J.; Shen, D.-F.; Chen, L.; Yan, L.; Tang, Q.; Li, H. Cellular repressor of E1A-stimulated genes attenuates cardiac hypertrophy and fibrosis. J. Cell. Mol. Med. 2009, 13, 1302–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.-Y.; Zhao, L.-P.; Tian, X.-X.; Yan, C.-H.; Li, Y.; Liu, Y.; Wang, P.; Zhang, X.-J.; Han, Y. The novel intracellular protein CREG inhibits hepatic steatosis, obesity, and insulin resistance. Hepatology 2017, 66, 834–854. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Yan, C.; Liu, M.; Zhang, Q.; Liu, D.; Liu, Y.; Li, S.; Han, Y. CREG1 heterozygous mice are susceptible to high fat diet-induced obesity and insulin resistance. PLoS ONE 2017, 12, e0176873. [Google Scholar] [CrossRef] [PubMed]
- Ben-Porath, I.; Weinberg, R.A. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 2005, 37, 961–976. [Google Scholar] [CrossRef]
- Sherr, C.J. Divorcing ARF and p53: An unsettled case. Nat. Rev. Cancer 2006, 6, 663–673. [Google Scholar] [CrossRef]
- Campisi, J.; Di Fagagna, F.D. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Kuilman, T.; Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 2009, 9, 81–94. [Google Scholar] [CrossRef]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; Lebrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.-M.; et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nat. Cell Biol. 2016, 530, 184–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, M.; Asai, A.; Kawagishi, H.; Mikawa, R.; Iwashita, Y.; Kanayama, K.; Sugimoto, K.; Sato, T.; Maruyama, M.; Sugimoto, M. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight 2016, 1, e87732. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Peng, C.-Y.; Lin, S.-Y.; Qin, Z.-Y.; Liang, J.-L.; Chen, H.-J.; Hou, C.-X.; Wang, R.; Du, Y.-Q.; Jin, J.-L.; et al. P16INK4a played a critical role in exacerbating acute tubular necrosis in acute kidney injury. Am. J. Transl. Res. 2019, 11, 3850–3861. [Google Scholar]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.-E.; Kozak, L.P. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nat. Cell Biol. 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Kozak, L.P.; Harper, M.-E. Mitochondrial uncoupling proteins in energy expenditure. Annu. Rev. Nutr. 2000, 20, 339–363. [Google Scholar] [CrossRef]
- Kontani, Y.; Wang, Y.; Kimura, K.; Inokuma, K.-I.; Saito, M.; Suzuki-Miura, T.; Wang, Z.; Sato, Y.; Mori, N.; Yamashita, H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell 2005, 4, 147–155. [Google Scholar] [CrossRef]
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef] [Green Version]
- Sugino, T.; Okada, A.; Taguchi, K.; Unno, R.; Hamamoto, S.; Ando, R.; Mogami, T.; Kohri, K.; Yamashita, H.; Yasui, T. Brown adipocytes and β3-stimulant-induced brown-like adipocytes contribute to the prevention of renal crystal formation. Am. J. Physiol. Physiol. 2019, 316, F1282–F1292. [Google Scholar] [CrossRef]
- Kusudo, T.; Hashimoto, M.; Kataoka, N.; Li, Y.; Nozaki, A.; Yamashita, H. CREG1 promotes uncoupling protein 1 expression and brown adipogenesisin vitro. J. Biochem. 2018, 165, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kusudo, T.; Takeuchi, T.; Kataoka, N.; Mukai, T.; Yamashita, H. CREG1 stimulates brown adipocyte formation and ameliorates diet-induced obesity in mice. FASEB J. 2019, 33, 8069–8082. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Sheaff, M.T. Renal ageing. J. Pathol. 2007, 211, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.; Rule, A.D. The implications of anatomical and functional changes of the aging kidney: With an emphasis on the glomeruli. Kidney Int. 2012, 82, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Adler, S. Structure-function relationships associated with extracellular matrix alterations in diabetic glomerulopathy. J. Am. Soc. Nephrol. 1994, 5, 1165–1172. [Google Scholar] [PubMed]
- Genovese, F.; Manresa, A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Leelahavanichkul, A.; Souza, A.C.P.; Street, J.M.; Hsu, V.; Tsuji, T.; Doi, K.; Li, L.; Hu, X.; Zhou, H.; Kumar, P.; et al. Comparison of serum creatinine and serum cystatin C as biomarkers to detect sepsis-induced acute kidney injury and to predict mortality in CD-1 mice. Am. J. Physiol. Physiol. 2014, 307, F939–F948. [Google Scholar] [CrossRef] [Green Version]
- Bulavin, D.V.; Phillips, C.; Nannenga, B.; Timofeev, O.; Donehower, L.A.; Anderson, C.W.; Appella, E., Jr.; Fornace, A.J. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK–mediated activation of the p16Ink4a-p19Arf pathway. Nat. Genet. 2004, 36, 343–350. [Google Scholar] [CrossRef]
- Thornton, T.M.; Rincon, M. Non-Classical P38 Map Kinase Functions: Cell Cycle Checkpoints and Survival. Int. J. Biol. Sci. 2009, 5, 44–52. [Google Scholar] [CrossRef]
- Yamashita, H.; Kusudo, T.; Takeuchi, T.; Qiao, S.; Tsutsumiuchi, K.; Wang, T.; Wang, Y. Dietary supplementation with evodiamine prevents obesity and improves insulin resistance in ageing mice. J. Funct. Foods 2015, 19, 320–329. [Google Scholar] [CrossRef]
- Yamashita, H.; Yamamoto, M.; Ookawara, T.; Sato, Y.; Ueno, N.; Ohno, H. Discordance Between Thermogenic Activity and Expression of Uncoupling Protein in Brown Adipose Tissue of Old Rats. J. Gerontol. 1994, 49, B54–B59. [Google Scholar] [CrossRef] [PubMed]
- Gabaldon, A.M.; Florez-Duquet, M.L.; Hamilton, J.S.; McDonald, R.B.; Horwitz, B.A. Effects of age and gender on brown fat and skeletal muscle metabolic responses to cold in F344 rats. Am. J. Physiol. Integr. Comp. Physiol. 1995, 268, R931–R941. [Google Scholar] [CrossRef]
- Krishnamurthy, J.; Torrice, C.; Ramsey, M.R.; Kovalev, G.I.; Al-Regaiey, K.; Su, L.; Sharpless, N.E. Ink4a/Arf expression is a biomarker of aging. J. Clin. Investig. 2004, 114, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Palmer, A.K.; Ding, H.; Weivoda, M.M.; Pirtskhalava, T.; White, T.A.; Sepe, A.; Johnson, K.O.; Stout, M.B.; Giorgadze, N.; et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife 2015, 4, e12997. [Google Scholar] [CrossRef] [PubMed]
- De Magalhães, J.P.; Passos, J.F. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 2018, 170, 2–9. [Google Scholar] [CrossRef]
- Ma, F.Y.; Liu, J.; Nikolic-Paterson, D.J. The role of stress-activated protein kinase signaling in renal pathophysiology. Braz. J. Med. Biol. Res. 2008, 42, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Jin, N.; Wang, Q.; Zhang, X.; Jiang, D.; Cheng, H.; Zhu, K. The selective p38 mitogen-activated protein kinase inhibitor, SB203580, improves renal disease in MRL/lpr mouse model of systemic lupus. Int. Immunopharmacol. 2011, 11, 1319–1326. [Google Scholar] [CrossRef]
- Wang, D.; Warner, G.M.; Yin, P.; Knudsen, B.E.; Cheng, J.; Butters, K.A.; Lien, K.R.; Gray, C.E.; Garovic, V.D.; Lerman, L.O.; et al. Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in a murine renal artery stenosis model. Am. J. Physiol. Physiol. 2013, 304, F938–F947. [Google Scholar] [CrossRef] [Green Version]
- Freund, A.; Orjalo, A.V.; Desprez, P.-Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Offen, D.; Barhum, Y.; Melamed, E.; Embacher, N.; Schindler, C.; Ransmayr, G. Spinal Cord mRNA Profile in Patients with ALS: Comparison with Transgenic Mice Expressing the Human SOD-1 Mutant. J. Mol. Neurosci. 2008, 38, 85–93. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, M.; Goto, A.; Endo, Y.; Sugimoto, M.; Ueda, J.; Yamashita, H. Effects of CREG1 on Age-Associated Metabolic Phenotypes and Renal Senescence in Mice. Int. J. Mol. Sci. 2021, 22, 1276. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031276
Hashimoto M, Goto A, Endo Y, Sugimoto M, Ueda J, Yamashita H. Effects of CREG1 on Age-Associated Metabolic Phenotypes and Renal Senescence in Mice. International Journal of Molecular Sciences. 2021; 22(3):1276. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031276
Chicago/Turabian StyleHashimoto, Michihiro, Ayumi Goto, Yuki Endo, Masataka Sugimoto, Jun Ueda, and Hitoshi Yamashita. 2021. "Effects of CREG1 on Age-Associated Metabolic Phenotypes and Renal Senescence in Mice" International Journal of Molecular Sciences 22, no. 3: 1276. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031276