Effect of Aminated Chitosan-Coated Fe3O4 Nanoparticles with Applicational Potential in Nanomedicine on DPPG, DSPC, and POPC Langmuir Monolayers as Cell Membrane Models
Abstract
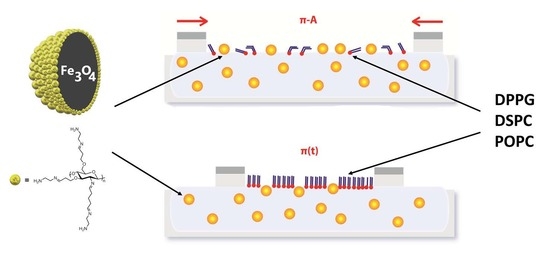
:1. Introduction
- 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC),
- 1,2-dipalmitoyl-sn-glycero-3-phospho-rac-(1-glycerol) (DPPG), and
- 2-oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (POPC).
2. Results and Discussion
2.1. Phospholipid Films Compressed on the Water Subphase Containing the Fe3O4-AChit Nanoparticles
2.1.1. DPPG Compressed on the Water Subphases with the Different Concentrations of the Fe3O4-AChit Nanoparticles
2.1.2. DSPC Compressed on the Water Subphases with the Different Concentrations of the Fe3O4-AChit Nanoparticles
2.1.3. POPC Compressed on the Water Subphases with the Different Concentrations of the Fe3O4-AChit Nanoparticles
2.2. Adsorption Kinetics of the Fe3O4-AChit Nanoparticles to the Phospholipid Monolayers
3. Materials and Methods
3.1. Materials
3.1.1. Synthesis and Characterization of the Fe3O4-AChit Nanoparticles
3.1.2. Phospholipids
3.2. Methods
3.2.1. Preparation of the Water Suspensions of the Fe3O4-AChit Nanoparticles
3.2.2. Compression of the Phospholipid Monolayers in the Subphase Containing the Fe3O4-AChit Nanoparticles
3.2.3. Brewster Angle Microscopy Observations
3.2.4. Penetration Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Seo, J.; Yang, C.; Prasad, P.N. Nanochemistry and nanomaterials for photovoltaics. Chem. Soc. Rev. 2013, 42, 8304–8338. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef]
- Xu, R.-P.; Li, Y.-Q.; Tang, J.-X. Recent advances in flexible organic light-emitting diodes. J. Mater. Chem. C 2016, 4, 9116–9142. [Google Scholar] [CrossRef]
- Kędzierski, K.; Rytel, K.; Barszcz, B.; Gronostaj, A.; Majchrzycki, Ł.; Wróbel, D. Unusual conductivity temperature dependence of multiwalled carbon nanotube thin film. Chem. Phys. Lett. 2018, 712, 144–148. [Google Scholar] [CrossRef]
- Qian, H.; Liu, B.; Jiang, X. Application of nanomaterials in cancer immunotherapy. Mater. Today Chem. 2018, 7, 53–64. [Google Scholar] [CrossRef]
- Van der Heijden, A.E.D.M. Developments and challenges in the manufacturing, characterization and scale-up of energetic nanomaterials—A review. Chem. Eng. J. 2018, 350, 939–948. [Google Scholar] [CrossRef]
- El-Ahmar, S.; Koczorowski, W.; Poźniak, A.A.; Kuświk, P.; Przychodnia, M.; Dembowiak, J.; Strupiński, W. Planar configuration of extraordinary magnetoresistance for 2D-material-based magnetic field sensors. Sens. Actuators A Phys. 2019, 296, 249–253. [Google Scholar] [CrossRef]
- Szary, M.J. Bonding and electronics of the silicene/MoTe2 interface under strain. Appl. Surf. Sci. 2019, 491, 469–477. [Google Scholar] [CrossRef]
- Szary, M.J. Giant Rashba spin splitting induced by heavy element adsorption at germanene. FlatChem 2019, 18, 100141. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, K. Recent progress on graphene-analogous 2D nanomaterials: Properties, modeling and applications. Prog. Mater. Sci. 2019, 100, 99–169. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A. Engineered nanomaterials for water decontamination and purification: From lab to products. J. Hazard. Mater. 2019, 363, 295–308. [Google Scholar] [CrossRef]
- Abd Elkodous, M.; El-Sayyad, G.S.; Abdelrahman, I.Y.; El-Bastawisy, H.S.; Mohamed, A.E.; Mosallam, F.M.; Nasser, H.A.; Gobara, M.; Baraka, A.; Elsayed, M.A.; et al. Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf. B Biointerfaces 2019, 180, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Ding, W.; Kameta, N. Soft-matter nanotubes: A platform for diverse functions and applications. Chem. Rev. 2020, 120, 2347–2407. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Majidzadeh-A., K. Applications of two-dimensional nanomaterials in breast cancer theranostics. Acs Biomater. Sci. Eng. 2020, 6, 1852–1873. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, C.Z.; Roy, V.A.L. Designed synthesis and surface engineering strategies of magnetic iron oxide nanoparticles for biomedical applications. Nanoscale 2016, 8, 19421–19474. [Google Scholar] [CrossRef]
- Rossi, L.M.; Costa, N.J.S.; Silva, F.P.; Wojcieszak, R. Magnetic nanomaterials in catalysis: Advanced catalysts for magnetic separation and beyond. Green Chem. 2014, 16, 2906–2933. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef] [Green Version]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Basini, M.; Guerrini, A.; Cobianchi, M.; Orsini, F.; Bettega, D.; Avolio, M.; Innocenti, C.; Sangregorio, C.; Lascialfari, A.; Arosio, P. Tailoring the magnetic core of organic-coated iron oxides nanoparticles to influence their contrast efficiency for Magnetic Resonance Imaging. J. Alloy. Compd. 2019, 770, 58–66. [Google Scholar] [CrossRef]
- Roet, M.; Hescham, S.-A.; Jahanshahi, A.; Rutten, B.P.F.; Anikeeva, P.O.; Temel, Y. Progress in neuromodulation of the brain: A role for magnetic nanoparticles? Prog. Neurobiol. 2019, 177, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Fratila, R.M.; Rivera-Fernández, S.; de la Fuente, J.M. Shape matters: Synthesis and biomedical applications of high aspect ratio magnetic nanomaterials. Nanoscale 2015, 7, 8233–8260. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The role of ligands in the chemical synthesis and applications of inorganic nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rascol, E.; Devoisselle, J.M.; Chopineau, J. The relevance of membrane models to understand nanoparticles-cell membrane interactions. Nanoscale 2016, 8, 4780–4798. [Google Scholar] [CrossRef] [PubMed]
- Neunert, G.; Makowiecki, J.; Piosik, E.; Hertmanowski, R.; Polewski, K.; Martynski, T. Miscibility of dl-α-tocopherol β-glucoside in DPPC monolayer at air/water and air/solid interfaces. Mater. Sci. Eng. C 2016, 67, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Caseli, L. Adsorption and enzyme activity of sucrose phosphorylase on lipid Langmuir and Langmuir–Blodgett films. Colloids Surf. B Biointerfaces 2014, 116, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Lhor, M.; Bernier, S.C.; Horchani, H.; Bussières, S.; Cantin, L.; Desbat, B.; Salesse, C. Comparison between the behavior of different hydrophobic peptides allowing membrane anchoring of proteins. Adv. Colloid Interface Sci. 2014, 207, 223–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavinatto, F.J.; Caseli, L.; Pavinatto, A.; dos Santos David, S.; Nobre, T.M.; Zaniquelli, M.E.D.; Silva, H.S.; Miranda, P.B.; de Oliveira, O.N. Probing chitosan and phospholipid interactions using Langmuir and Langmuir−Blodgett films as cell membrane models. Langmuir 2007, 23, 7666–7671. [Google Scholar] [CrossRef]
- Liu, W.; Johnson, S.; Micic, M.; Orbulescu, J.; Whyte, J.; Garcia, A.R.; Leblanc, R.M. Study of the aggregation of human insulin Langmuir monolayer. Langmuir 2012, 28, 3369–3377. [Google Scholar] [CrossRef]
- Thakur, G.; Micic, M.; Leblanc, R.M. Surface chemistry of Alzheimer’s disease: A Langmuir monolayer approach. Colloids Surf. B Biointerfaces 2009, 74, 436–456. [Google Scholar] [CrossRef]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef]
- Nobre, T.M.; Pavinatto, F.J.; Caseli, L.; Barros-Timmons, A.; Dynarowicz-Łątka, P.; Oliveira Jr, O.N. Interactions of bioactive molecules & nanomaterials with Langmuir monolayers as cell membrane models. Thin Solid Film. 2015, 593, 158–188. [Google Scholar] [CrossRef]
- Butt, H.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; John Wiley and Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gaines, G.L. Insoluble Monolayers at Liquid-Gas Interfaces; Interscience Publishers: New York, NY, USA, 1966. [Google Scholar]
- Marszałł, M.P.; Sroka, W.D.; Sikora, A.; Chełminiak, D.; Ziegler-Borowska, M.; Siódmiak, T.; Moaddel, R. Ligand fishing using new chitosan based functionalized Androgen Receptor magnetic particles. J. Pharm. Biomed. Anal. 2016, 127, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, A.; Chełminiak-Dudkiewicz, D.; Siódmiak, T.; Tarczykowska, A.; Sroka, W.D.; Ziegler-Borowska, M.; Marszałł, M.P. Enantioselective acetylation of (R,S)-atenolol: The use of Candida rugosa lipases immobilized onto magnetic chitosan nanoparticles in enzyme-catalyzed biotransformation. J. Mol. Catal. B Enzym. 2016, 134, 43–50. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Siódmiak, T.; Chełminiak, D.; Cyganiuk, A.; Marszałł, M.P. Magnetic nanoparticles with surfaces modified with chitosan–poly[N-benzyl-2-(methacryloxy)-N,N-dimethylethanaminium bromide] for lipase immobilization. Appl. Surf. Sci. 2014, 288, 641–648. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Siódmiak, T.; Sikora, A.; Piotr Marszałł, M.; Kaczmarek, H. Synthesis of new chitosan coated magnetic nanoparticles with surface modified with long-distanced amino groups as a support for bioligands binding. Mater. Lett. 2014, 132, 63–65. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Mylkie, K.; Kozlowska, M.; Nowak, P.; Chelminiak-Dudkiewicz, D.; Kozakiewicz, A.; Ilnicka, A.; Kaczmarek-Kedziera, A.; Ziegler-Borowska, M.; Mylkie, K.; et al. Effect of geometrical structure, drying, and synthetic method on aminated chitosan-coated magnetic nanoparticles utility for HSA effectiveimmobilization. Molecules 2019, 24, 1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piosik, E.; Klimczak, P.; Ziegler-Borowska, M.; Chełminiak-Dudkiewicz, D.; Martyński, T. A detailed investigation on interactions between magnetite nanoparticles functionalized with aminated chitosan and a cell model membrane. Mater. Sci. Eng. C 2020, 109, 110616. [Google Scholar] [CrossRef]
- Hao, C.; Li, J.; Mu, W.; Zhu, L.; Yang, J.; Liu, H.; Li, B.; Chen, S.; Sun, R. Adsorption behavior of magnetite nanoparticles into the DPPC model membranes. Appl. Surf. Sci. 2016, 362, 121–125. [Google Scholar] [CrossRef]
- Vollhardt, D.; Fainerman, V.B.; Siegel, S. Thermodynamic and textural characterization of DPPG phospholipid monolayers. J. Phys. Chem. B 2000, 104, 4115–4121. [Google Scholar] [CrossRef]
- Wydro, P.; Flasiński, M.; Broniatowski, M. Molecular organization of bacterial membrane lipids in mixed systems—A comprehensive monolayer study combined with Grazing Incidence X-ray Diffraction and Brewster Angle Microscopy experiments. Biochim. Biophys. Acta Biomembr. 2012, 1818, 1745–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrano, A.A.; Pereira, Â.S.; Oliveira, O.N.; Barros-Timmons, A. Probing the interaction of oppositely charged gold nanoparticles with DPPG and DPPC Langmuir monolayers as cell membrane models. Colloids Surf. B Biointerfaces 2013, 108, 120–126. [Google Scholar] [CrossRef]
- Matshaya, T.J.; Lanterna, A.E.; Granados, A.M.; Krause, R.W.M.; Maggio, B.; Vico, R. V Distinctive interactions of oleic acid covered magnetic nanoparticles with saturated and unsaturated phospholipids in Langmuir monolayers. Langmuir 2014, 30, 5888–5896. [Google Scholar] [CrossRef]
- Arnold, A.; Cloutier, I.; Ritcey, A.M.; Auger, M. Temperature and pressure dependent growth and morphology of DMPC/DSPC domains studied by Brewster angle microscopy. Chem. Phys. Lipids 2005, 133, 165–179. [Google Scholar] [CrossRef]
- Mogilevsky, A.; Volinsky, R.; Dayagi, Y.; Markovich, N.; Jelinek, R. Gold nanoparticle self-assembly in saturated phospholipid monolayers. Langmuir 2010, 26, 7893–7898. [Google Scholar] [CrossRef]
- Uehara, T.M.; Marangoni, V.S.; Pasquale, N.; Miranda, P.B.; Lee, K.-B.; Zucolotto, V. A detailed investigation on the interactions between magnetic nanoparticles and cell membrane models. Acs Appl. Mater. Interfaces 2013, 5, 13063–13068. [Google Scholar] [CrossRef]
- Bothun, G.D.; Ganji, N.; Khan, I.A.; Xi, A.; Bobba, C. Anionic and cationic silver nanoparticle binding restructures net-anionic PC/PG monolayers with saturated or unsaturated lipids. Langmuir 2017, 33, 51. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, S.S. Effects of lipid chain length on molecular interactions between paclitaxel and phospholipid within model biomembranes. J. Colloid Interface Sci. 2004, 274, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hao, C.; Xie, B.; Sun, R.; Stylianou, A. Effect of Fe3O 4 Nanoparticles on Mixed POPC/DPPC Monolayers at Air-Water Interface. Scanning 2019. [Google Scholar] [CrossRef] [PubMed]
- Grauby-Heywang, C.; Moroté, F.; Mathelié-Guinlet, M.; Gammoudi, I.; Faye, N.R.; Cohen-Bouhacina, T. Influence of oxidized lipids on palmitoyl-oleoyl-phosphatidylcholine organization, contribution of Langmuir monolayers and Langmuir–Blodgett films. Chem. Phys. Lipids 2016, 200, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Ziegler-Borowska, M.; Chelminiak-Dudkiewicz, D.; Siódmiak, T.; Sikora, A.; Wegrzynowska-Drzymalska, K.; Skopinska-Wisniewska, J.; Kaczmarek, H.; Marszałł, M. Chitosan–collagen coated magnetic nanoparticles for lipase immobilization—New type of “enzyme friendly” polymer shell crosslinking with squaric acid. Catalysts 2017, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H. Thermal stability of magnetic nanoparticles coated by blends of modified chitosan and poly(quaternary ammonium) salt. J.. Anal. Calorim. 2015, 119, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Chełminiak-Dudkiewicz, D.; Ziegler-Borowska, M.; Stolarska, M.; Sobotta, L.; Falkowski, M.; Mielcarek, J.; Goslinski, T.; Kowalonek, J.; Węgrzynowska-Drzymalska, K.; Kaczmarek, H. The chitosan—Porphyrazine hybrid materials and their photochemical properties. J. Photochem. Photobiol. B Biol. 2018, 181, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.T.; Rideal, S.E.K. Interfacial Phenomena; Academic Press: Cambridge, MA, USA, 1963. [Google Scholar]
- Hoenig, D.; Moebius, D. Direct visualization of monolayers at the air-water interface by Brewster angle microscopy. J. Phys. Chem. 1991, 95, 4590–4592. [Google Scholar] [CrossRef]
- Inglot, K.; Martyński, T.; Bauman, D. Influence of the alkyl chain length of some mesogenic molecules on their Langmuir film formation ability. Liq. Cryst. 2006, 33, 855–864. [Google Scholar] [CrossRef]
- Ábrahám, N.; Csapó, E.; Bohus, G.; Dékány, I. Interaction of biofunctionalized gold nanoparticles with model phospholipid membranes. Colloid Polym. Sci. 2014, 292, 2715–2725. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piosik, E.; Ziegler-Borowska, M.; Chełminiak-Dudkiewicz, D.; Martyński, T. Effect of Aminated Chitosan-Coated Fe3O4 Nanoparticles with Applicational Potential in Nanomedicine on DPPG, DSPC, and POPC Langmuir Monolayers as Cell Membrane Models. Int. J. Mol. Sci. 2021, 22, 2467. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052467
Piosik E, Ziegler-Borowska M, Chełminiak-Dudkiewicz D, Martyński T. Effect of Aminated Chitosan-Coated Fe3O4 Nanoparticles with Applicational Potential in Nanomedicine on DPPG, DSPC, and POPC Langmuir Monolayers as Cell Membrane Models. International Journal of Molecular Sciences. 2021; 22(5):2467. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052467
Chicago/Turabian StylePiosik, Emilia, Marta Ziegler-Borowska, Dorota Chełminiak-Dudkiewicz, and Tomasz Martyński. 2021. "Effect of Aminated Chitosan-Coated Fe3O4 Nanoparticles with Applicational Potential in Nanomedicine on DPPG, DSPC, and POPC Langmuir Monolayers as Cell Membrane Models" International Journal of Molecular Sciences 22, no. 5: 2467. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052467