Examination of Surfactant Protein D as a Biomarker for Evaluating Pulmonary Toxicity of Nanomaterials in Rat
Abstract
:1. Introduction
2. Results
2.1. Pathological Features in the Rat Lung
2.2. SP-D Concentrations in BALF
2.3. Correlation between SP-D and Inflammatory Markers in BALF
2.4. Gene Expression Analysis in Lung Tissue
2.5. Immunostaining of SP-D in Lung
2.6. SP-D Concentrations in Serum
2.7. The Relationship between SP-D Concentration in BALF and Serum
2.8. Assessment of the Accuracy of SP-D in BALF and Serum for Measuring the Toxicity of Nanomaterials
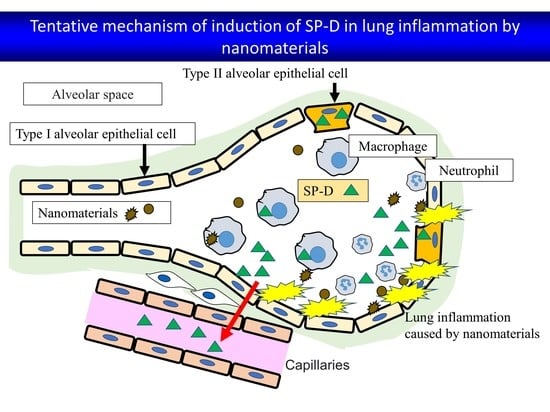
3. Discussion
4. Materials and Methods
4.1. Sample Nanomaterials
4.2. Animals
4.3. Intratracheal Instillation
4.4. Animals Following Intratracheal Instillation
4.5. Analysis of Inflammatory Cells in BALF with Cytospin
4.6. Measurement of SP-D, Chemokines, Lactate Dehydrogenase and Heme Oxygenase-1 in BALF
4.7. Total RNA Extraction
4.8. Validation of Gene Expression Data Using Quantitative Real-Time Polymerase Chain Reaction
4.9. Histopathology
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ogami, A.; Yamamoto, K.; Morimoto, Y.; Fujita, K.; Hirohashi, M.; Oyabu, T.; Myojo, T.; Nishi, K.; Kadoya, C.; Todoroki, M.; et al. Pathological features of rat lung following inhalation and intratracheal instillation of C60 fullerene. Inhal. Toxicol. 2011, 23, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Kuroda, E. Significance of Persistent Inflammation in Respiratory Disorders Induced by Nanoparticles. J. Immunol. Res. 2014, 2014, 962871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borm, P.J.; Driscoll, K. Particles, inflammation and respiratory tract carcinogenesis. Toxicol. Lett. 1996, 88, 109–113. [Google Scholar]
- Kim, H.; Morimoto, Y.; Ogami, A.; Nagatomo, H.; Hirohashi, M.; Oyabu, T.; Kawanami, Y.; Kuroda, E.; Higashi, T.; Tanaka, I. Differential expression of EC-SOD, Mn-SOD and CuZn-SOD in rat lung exposed to crystalline silica. J. Occup. Health 2007, 49, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Sayes, C.M.; Marchione, A.A.; Reed, K.L.; Warheit, D.B. Comparative Pulmonary Toxicity Assessments of C60 Water Suspensions in Rats: Few Differences in Fullerene Toxicity in Vivo in Contrast to in Vitro Profiles. Nano Lett. 2007, 7, 2399–2406. [Google Scholar] [CrossRef]
- Warheit, D.B.; Hoke, R.A.; Finlay, C.; Donner, E.M.; Reed, K.L.; Sayes, C.M. Development of a base set of toxicity tests using ultrafine TiO2 particles as a component of nanoparticle risk management. Toxicol. Lett. 2007, 171, 99–110. [Google Scholar] [CrossRef]
- Wright, J.R. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 2005, 5, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Sano, H.; Kuroki, Y. The lung collectins, SP-A and SP-D, modulate pulmonary innate immunity. Mol. Immunol. 2005, 42, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.R.; Williams, M.C.; Benson, B. Immunocytochemical localization of the major surfactant apoproteins in type II cells, Clara cells, and alveolar macrophages of rat lung. J. Histochem. Cytochem. 1986, 34, 1137–1148. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Omae, K.; Tanaka, A.; Hirata, M.; Michikawa, T.; Kikuchi, Y.; Yoshioka, N.; Nishiwaki, Y.; Chonan, T. Causal Relationship between Indium Compound Inhalation and Effects on the Lungs. J. Occup. Health 2009, 51, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, K.E.; Wright, J.R.; Steinberg, K.P.; Ruzinski, J.T.; Caldwell, E.; Wong, W.B.; Hull, W.; Whitsett, J.A.; Akino, T.; Kuroki, Y.; et al. Serial Changes in Surfactant-associated Proteins in Lung and Serum before and after Onset of ARDS. Am. J. Respir. Crit. Care Med. 1999, 160, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Sano, H.; Chiba, H.; Kuroki, Y. Pulmonary Surfactant Proteins A and D: Innate Immune Functions and Biomarkers for Lung Diseases. Curr. Pharm. Des. 2006, 12, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, C.; Lee, B.-W.; Ogami, A.; Oyabu, T.; Nishi, K.; Yamamoto, M.; Todoroki, M.; Morimoto, Y.; Tanaka, I.; Myojo, T. Analysis of pulmonary surfactant in rat lungs after inhalation of nanomaterials: Fullerenes, nickel oxide and multi-walled carbon nanotubes. Nanotoxicology 2016, 10, 194–203. [Google Scholar] [CrossRef]
- Okada, T.; Lee, B.W.; Ogami, A.; Oyabu, T.; Myojo, T. Inhalation of titanium dioxide (P25) nanoparticles to rats and changes in surfactant protein (SP-D) levels in bronchoalveolar lavage fluid and serum. Nanotoxicology 2019, 13, 1396–1408. [Google Scholar] [CrossRef]
- Gaunsbaek, M.Q.; Rasmussen, K.J.; Beers, M.F.; Atochina-Vasserman, E.N.; Hansen, S. Lung Surfactant Protein D (SP-D) Response and Regulation During Acute and Chronic Lung Injury. Lung 2013, 191, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Otsuka, M.; Ashida, N.; Yamada, G.; Kuronuma, K.; Chiba, H.; Takahashi, H. Surfactant protein D is a useful biomarker for monitoring acute lung injury in rats. Exp. Lung Res. 2016, 42, 314–321. [Google Scholar] [CrossRef]
- Pan, T.; Nielsen, L.D.; Allen, M.J.; Shannon, K.M.; Shannon, J.M.; Selman, M.; Mason, R.J. Serum SP-D is a marker of lung injury in rats. Am. J. Physiol. Cell. Mol. Physiol. 2002, 282, L824–L832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, M.; Shannon, J.M.; Ouchi, H.; Voelker, D.R.; Nakanishi, Y.; Mason, R.J. Serum surfactant protein D is increased in acute and chronic inflammation in mice. Cytokine 2005, 31, 25–33. [Google Scholar] [CrossRef]
- Takahashi, H.; Shiratori, M.; Kanai, A.; Chiba, H.; Kuroki, Y.; Abe, S. Monitoring markers of disease activity for interstitial lung diseases with serum surfactant proteins A and D. Respirology 2006, 11, S51–S54. [Google Scholar] [CrossRef]
- Yoshiura, Y.; Fujisawa, Y.; Tomonaga, T.; Izumi, H.; Oyabu, T.; Myojo, T.; Kubo, M.; Shimada, M.; Morimoto, Y. Comparison of Responses in Rat Lung Following Inhalation and Intratracheal Administration of Nanoparticles. In In Vivo Inhalation Toxicity Screening Methods for Manufactured Nanomaterials; Springer: Singapore, 2019; pp. 95–105. [Google Scholar]
- Tomonaga, T.; Izumi, H.; Yoshiura, Y.; Myojo, T.; Oyabu, T.; Lee, B.-W.; Okada, T.; Marui, T.; Wang, K.-Y.; Kubo, M.; et al. Usefulness of myeloperoxidase as a biomarker for the ranking of pulmonary toxicity of nanomaterials. Part. Fibre Toxicol. 2018, 15, 41. [Google Scholar] [CrossRef] [Green Version]
- Tomonaga, T.; Izumi, H.; Oyabu, T.; Lee, B.-W.; Kubo, M.; Shimada, M.; Noguchi, S.; Nishida, C.; Yatera, K.; Morimoto, Y. Assessment of Cytokine-Induced Neutrophil Chemoattractants as Biomarkers for Prediction of Pulmonary Toxicity of Nanomaterials. Nanomaterials 2020, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Signal Detection Theory and ROC Analysis in Psychology and Diagnostics: Collected Papers; Psychology Press: Hove, UK, 1996; p. 324. ISBN 0805818340. [Google Scholar]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Lee, B.-W.; Okada, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; et al. Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanoparticles. Nanotoxicology 2016, 10, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Pulmonary toxicity of well-dispersed cerium oxide nanoparticles following intratracheal instillation and inhalation. J. Nanopart. Res. 2015, 17, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Tomonaga, T.; Oyabu, T.; Myojo, T.; Kawai, K.; Yatera, K.; Shimada, M.; Kubo, M.; et al. Evaluation of pulmonary toxicity of zinc oxide nanoparticles following inhalation and intratracheal instillation. Int. J. Mol. Sci. 2016, 17, 1241. [Google Scholar] [CrossRef] [PubMed]
- Yoshiura, Y.; Izumi, H.; Oyabu, T.; Hashiba, M.; Kambara, T.; Mizuguchi, Y.; Lee, B.W.; Okada, T.; Tomonaga, T.; Myojo, T.; et al. Pulmonary toxicity of well-dispersed titanium dioxide nanoparticles following intratracheal instillation. J. Nanopart. Res. 2015, 17, 241. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y.; Izumi, H.; Yoshiura, Y.; Fujishima, K.; Yatera, K.; Yamamoto, K. Usefulness of Intratracheal Instillation Studies for Estimating Nanoparticle-Induced Pulmonary Toxicity. Int. J. Mol. Sci. 2016, 17, 165. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, S.; Izumi, H.; Morimoto, Y.; Sakurai, K.; Mochizuki, S. Induction of potent cell growth inhibition by schizophyllan/K-ras antisense complex in combination with gemcitabine. Bioorg. Med. Chem. 2020, 28, 115668. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Izumi, H.; Tomonaga, T.; Takeshita, J.; Wang, K.-Y.; Yamasaki, K.; Yatera, K.; Morimoto, Y. Predictive Biomarkers for the Ranking of Pulmonary Toxicity of Nanomaterials. Nanomaterials 2020, 10, 2032. [Google Scholar] [CrossRef]









| Dose | TiO2 (P90) | TiO2 (rutile) | ZnO | NiO | CeO2 | SiO2 | |
|---|---|---|---|---|---|---|---|
| SP-D in BALF (ng/mL) | Control | 1644 ± 235 | 874 ± 79 | 997 ± 127 | 667 ± 271 | 754 ± 103 | 531 ± 126 |
| 0.2 mg | 2299 ± 394 | 1084 ± 349 | 986 ± 35 | 1519 ± 493 | 1440 ± 267 | - | |
| 1.0 mg | 2037 ± 632 | 1317 ± 717 | 829 ± 76 | 4852 ± 1036 | 3010 ± 61 | 2194 ± 253 | |
| Exposure/control ratio of BALF | 0.2 mg | 1.4 | 1.2 | 1.0 | 2.3 | 1.9 | - |
| 1.0 mg | 1.2 | 1.5 | 0.8 | 7.3 | 4.0 | 4.1 | |
| SP-D in serum (ng/mL) | Control | 84 ± 30 | - | - | 84 ± 6 | - | 112 ± 9 |
| 0.2 mg | 73 ± 8 | - | - | 127 ± 59 | - | - | |
| 1.0 mg | 85 ± 16 | - | - | 136 ± 18 | - | 136 ± 8 | |
| Exposure/control ratio of serum | 0.2 mg | 0.9 | - | - | 1.5 | - | - |
| 1.0 mg | 1.0 | - | - | 1.6 | - | 1.2 |
| Time | AUC | 95% CI | p Values | |
|---|---|---|---|---|
| SP-D in BALF | 3 days | 0.463 | 0.296–0.631 | 0.663 |
| 1 week | 0.803 | 0.674–0.933 | 0.000 | |
| 1 month | 0.982 | 0.952–1.000 | 0.000 | |
| 3 months | 0.795 | 0.670–0.920 | 0.000 | |
| 6 months | 0.717 | 0.567–0.866 | 0.010 | |
| SP-D in serum | 3 days | 0.424 | 0.144–0.703 | 0.597 |
| 1 week | 0.875 | 0.694–1.000 | 0.009 | |
| 1 month | 1.000 | 1.000–1.000 | 0.001 | |
| 3 months | 0.903 | 0.750–1.000 | 0.005 | |
| 6 months | 0.521 | 0.232–0.810 | 0.885 |
| Samples | Toxicity | Characterization | Animal (Rat) | Dose | Lung Inflammation | Reference |
|---|---|---|---|---|---|---|
| NiO | High | Size 19 nm, BET 57 m2/g Secondary particle diameter (DLS) 59.7 nm | Male Fischer 344 | 0.2 mg/rat, 1.0 mg/rat | + | [25] |
| CeO2 | High | Size 7.8 nm, BET 101 m2/g Secondary particle diameter (DLS) 10.0 nm | Male Fischer 344 | 0.2 mg/rat, 1.0 mg/rat | + | [26] |
| TiO2 (P90) | Low | Size 14 nm, BET 104 m2/g Secondary particle diameter (DLS) 22.7 nm | Male Wistar Hannover | 0.2 mg/rat, 1.0 mg/rat | − | [28] |
| TiO2 (rutile) | Low | Size 12 nm × 55 nm, BET 111 m2/g Secondary particle diameter (DLS) 44.9 nm | Male Fischer 344 | 0.2 mg/rat, 1.0 mg/rat | − | [25] |
| ZnO | Low | Size 35 nm, BET 31 m2/g Secondary particle diameter (DLS) 33 nm | Male Fischer 344 | 0.2 mg/rat, 1.0 mg/rat | − | [27] |
| SiO2 | High | Primary particle size 1.6 µm | Male Fischer 344 | 1.0 mg/rat | + | [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomonaga, T.; Izumi, H.; Yoshiura, Y.; Nishida, C.; Yatera, K.; Morimoto, Y. Examination of Surfactant Protein D as a Biomarker for Evaluating Pulmonary Toxicity of Nanomaterials in Rat. Int. J. Mol. Sci. 2021, 22, 4635. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094635
Tomonaga T, Izumi H, Yoshiura Y, Nishida C, Yatera K, Morimoto Y. Examination of Surfactant Protein D as a Biomarker for Evaluating Pulmonary Toxicity of Nanomaterials in Rat. International Journal of Molecular Sciences. 2021; 22(9):4635. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094635
Chicago/Turabian StyleTomonaga, Taisuke, Hiroto Izumi, Yukiko Yoshiura, Chinatsu Nishida, Kazuhiro Yatera, and Yasuo Morimoto. 2021. "Examination of Surfactant Protein D as a Biomarker for Evaluating Pulmonary Toxicity of Nanomaterials in Rat" International Journal of Molecular Sciences 22, no. 9: 4635. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094635