Preventive Applications of Polyphenols in Dentistry—A Review
Abstract
:1. Introduction
2. Polyphenols and Their Dietary Sources
3. Polyphenolic Tea Drugs in Dentistry
3.1. Classic Tea Drugs
3.1.1. Green Tea
3.1.2. Oolong Tea
3.1.3. Black Tea
3.2. Other Tea Drugs
3.2.1. Cistus Incanus Tea
3.2.2. Inula Viscosa Tea
3.2.3. Fragaria Vesca Tea
3.2.4. Hamamelis Virginiana Tea
3.2.5. Tormentil Tea
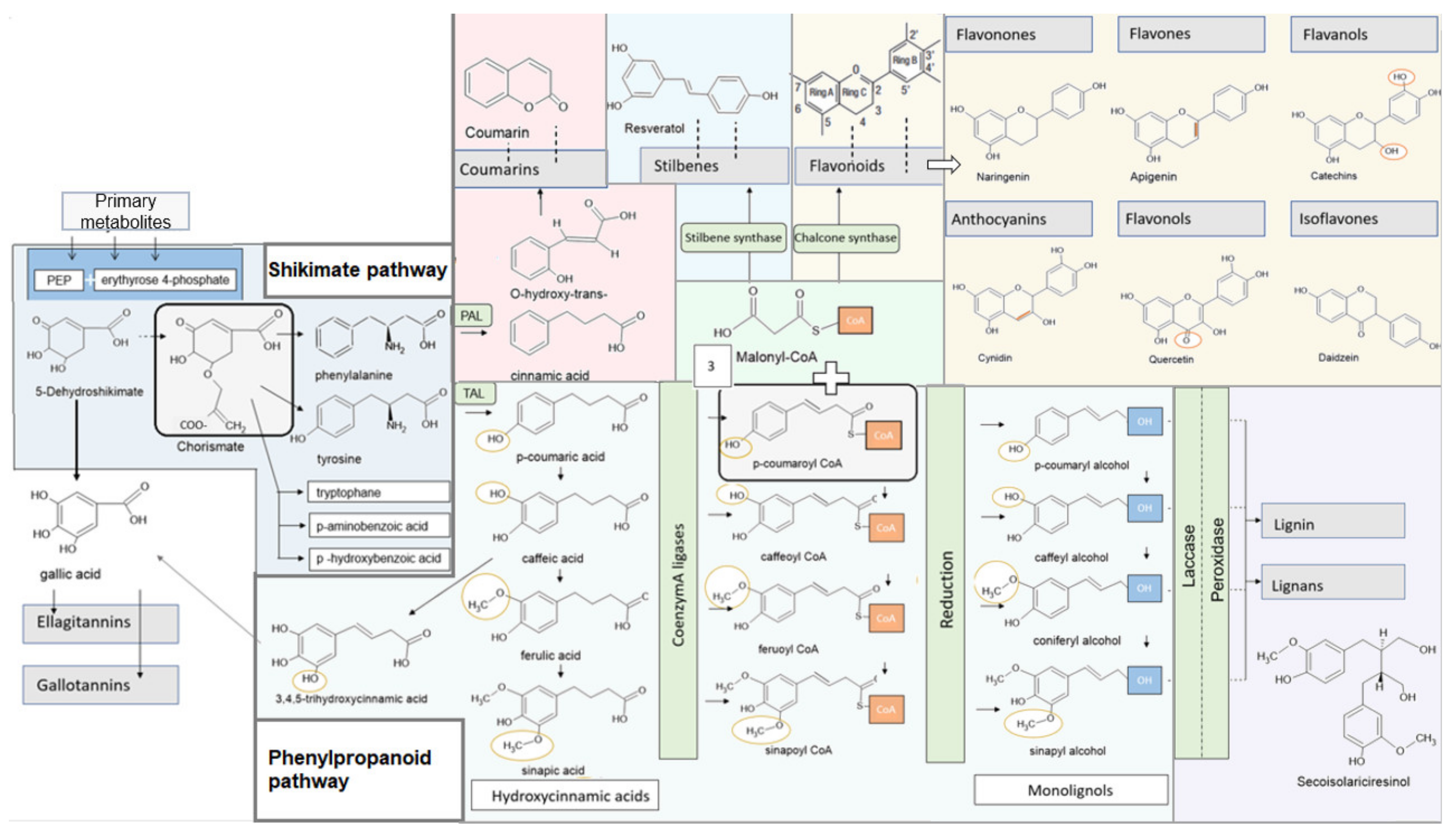
4. Metabolic Pathways in Higher Plants
4.1. Shikimate Pathway
4.2. Phenylpropanoid Synthesis and Structure of the Individual Phenylpropanoids
4.3. Specific Synthesis of Different Polyphenols
4.3.1. Lignans, Lignin
4.3.2. Gallic Acid, Hydrolysable Tannins: Ellagitannins, Gallotannins
4.3.3. Flavonoids, Stilbenes
4.3.4. Catechins and Theaflavins
4.3.5. Coumarins
5. Chemical, Physical and Biological Properties of Polyphenols
5.1. Chemical Properties
5.2. Physical Properties
5.3. Biological Properties
6. Pellicle Formation and Initial Bacterial Colonization on Solid Surfaces
6.1. Mucosal Pellicle
6.2. Acquired Enamel Pellicle
6.3. Polyphenols as Anti-Adherent Agents
7. Influence of Tea Polyphenols onto the Acquired Enamel Pellicle
7.1. Green Tea
7.2. Black Tea
7.3. Cistus Incanus Tea
7.4. Inula Viscosa Tea
7.5. Fragaria Vesca, Hamamelis and Tormentil Tea
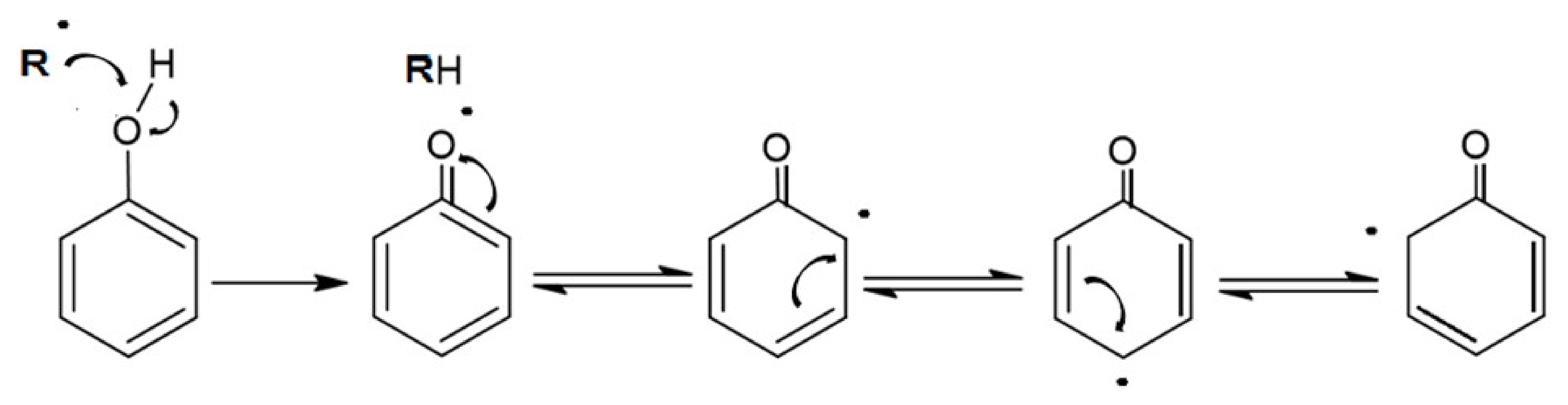
8. Mechanisms for Provided Effects by Polyphenols
8.1. Effect of Polyphenols on Oral Proteins
8.2. Effect of Polyphenolic Compounds on Bacteria
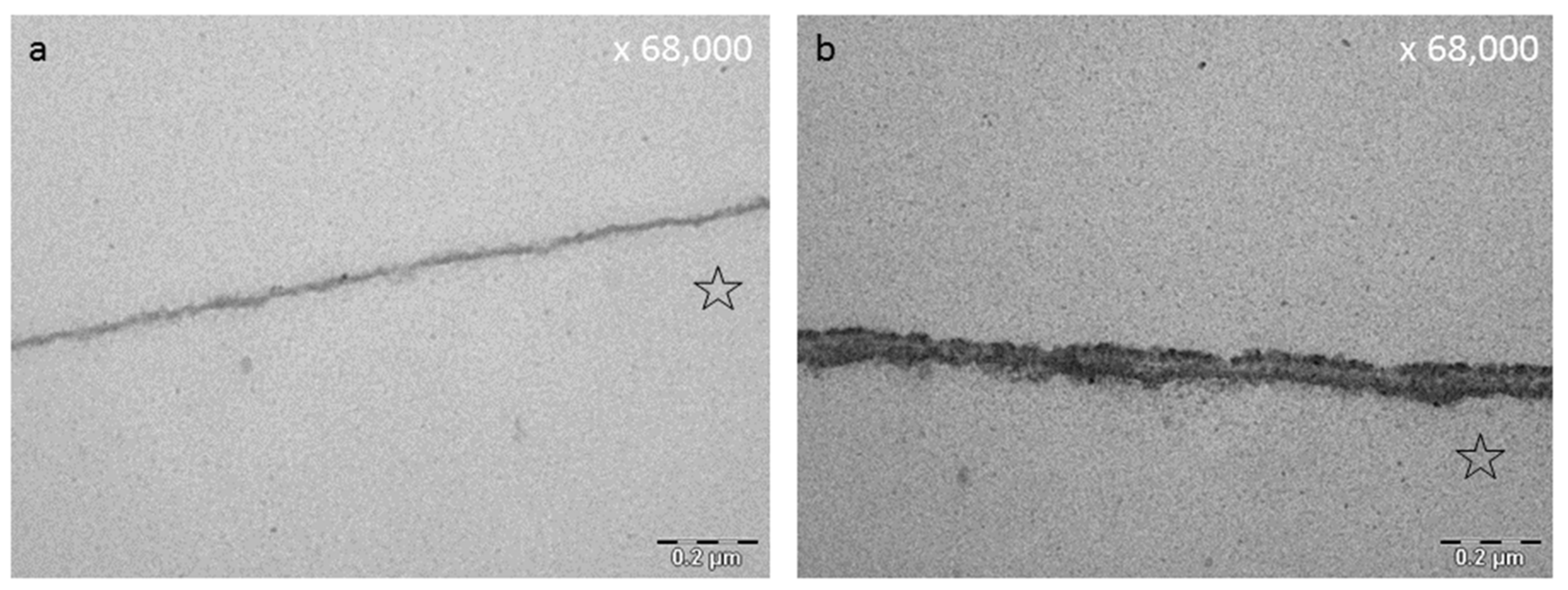
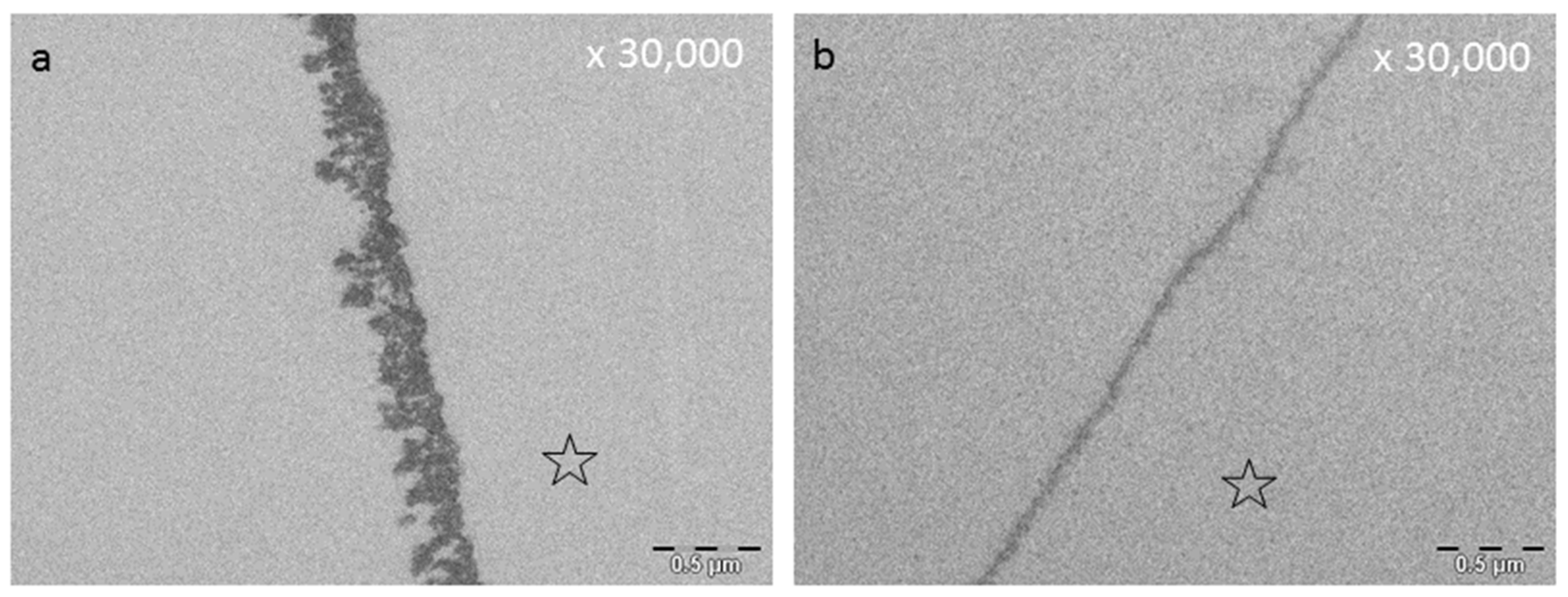
8.3. Effect of Tea Polyphenols on the Ultrastructure of the Pellicle
8.4. Influence of Tea Polyphenols on Erosive Attacks of the Enamel Surface
9. Discussion
10. Conclusion and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unachukwu, U.J.; Ahmed, S.; Kavalier, A.; Lyles, J.T.; Kennelly, E.J. White and Green Teas (Camellia sinensis var. sinensis): Variation in Phenolic, Methylxanthine, and Antioxidant Profiles. J. Food Sci. 2010, 75, C541–C548. [Google Scholar] [CrossRef] [Green Version]
- Ditano-Vazquez, P.; Torres-Pena, J.D.; Galeano-Valle, F.; Perez-Caballero, A.I.; Demelo-Rodriguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [Green Version]
- Niemetz, R.; Gross, G.G. Enzymology of gallotannin and ellagitannin biosynthesis. Phytochemistry 2005, 66, 2001–2011. [Google Scholar] [CrossRef]
- Hannig, C.; Attin, T.; Hannig, M.; Henze, E.; Brinkmann, K.; Zech, R. Immobilisation and activity of human alpha-amylase in the acquired enamel pellicle. Arch. Oral Biol. 2004, 49, 469–475. [Google Scholar] [CrossRef]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol. 2015, 60, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, K.; Parker, R.G. The United Nations High Level Meeting on the Prevention and Control of Noncommunicable Diseases: A Missed Opportunity? Am. J. Public Health 2012, 102, 2010–2012. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Woelber, J.P.; Bremer, K.; Vach, K.; Konig, D.; Hellwig, E.; Ratka-Kruger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2016, 16, 109. [Google Scholar] [CrossRef] [Green Version]
- Gondivkar, S.M.; Gadbail, A.R.; Gondivkar, R.S.; Sarode, S.C.; Sarode, G.S.; Patil, S.; Awan, K.H. Nutrition and oral health. Dis. Mon. 2019, 65, 147–154. [Google Scholar] [CrossRef]
- Bhattacharya, U.; Mukhopadhyay, S.; Giri, A.K. Comparative Antimutagenic and Anticancer Activity of Three Fractions of Black Tea Polyphenols Thearubigins. Nutr. Cancer 2011, 63, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, J.R.; Wyse, D.L.; Sheaffer, C.C.; Kauppi, K.L.; Fulcher, R.G.; Ehlke, N.J.; Biesboer, D.D.; Bey, R.F. Antioxidant and antimicrobial activity of seed from plants of the Mississippi river basin. J. Med. Plants Res. 2009, 3, 707–718. [Google Scholar]
- Bowden, G.H.W. Controlled environment model for accumulation of biofilms of oral bacteria. Method Enzymol. 1999, 310, 216–224. [Google Scholar]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant Polyphenols and Their Anti-Cariogenic Properties: A Review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.G.; Rosalen, P.L.; Falsetta, M.L.; Koo, H. Natural Products in Caries Research: Current (Limited) Knowledge, Challenges and Future Perspective. Caries Res. 2011, 45, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.L.; Kogan, M. Plant volatiles as insect attractants. Crit. Rev. Plant Sci. 1987, 5, 251–301. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentao, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Martens, S.; Mithofer, A. Flavones and flavone synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, I.L.F.; Chee, W.S.S.; Poulsen, L.; Offord-Cavin, E.; Rasmussen, S.E.; Frederiksen, H.; Enslen, M.; Barron, D.; Horcajada, M.N.; Williamson, G. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: A randomized, double-blind, crossover trial. J. Nutr. 2006, 136, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Bloedon, L.T.; Jeffcoat, A.R.; Lopaczynski, W.; Schell, M.J.; Black, T.M.; Dix, K.J.; Thomas, B.F.; Albright, C.; Busby, M.G.; Crowell, J.A.; et al. Safety and pharmacokinetics of purified soy isoflavones: Single-dose administration to postmenopausal women. Am. J. Clin. Nutr. 2002, 76, 1126–1137. [Google Scholar] [CrossRef] [Green Version]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Desai, P.B.; Zimmer-Nechimias, L.; Wolfe, B.; Jakate, A.S.; Creutzinger, V.; Heubi, J.E. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J. Nutr. 2003, 133, 1027–1035. [Google Scholar] [CrossRef] [Green Version]
- DuPont, M.S.; Mondin, Z.; Williamson, G.; Price, K.R. Effect of variety, processing, and storage on the flavonoid glycoside content and composition of lettuce and endive. J. Agric. Food Chem. 2000, 48, 3957–3964. [Google Scholar] [CrossRef]
- Van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M.F. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, biochemistry, and dietary role of potato polyphenols. A review. J. Agric. Food Chem. 1997, 45, 1523–1540. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Bohm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef]
- Theppakorn, T.; Luthfiyyah, A.; Ploysri, K. Comparison of the Composition and Antioxidant Capacities of Green Teas Produced from the Assam and the Chinese Varieties Cultivated in Thailand. J. Microbiol. Biotechnol. Food. 2014, 3, 364–370. [Google Scholar]
- Hosoda, K.; Wang, M.F.; Liao, M.L.; Chuang, C.K.; Iha, M.; Clevidence, B.; Yamamoto, S. Antihyperglycemic effect of oolong tea in type 2 diabetes. Diabetes Care 2003, 26, 1714–1718. [Google Scholar] [CrossRef] [Green Version]
- Ng, H.L.H.; Premilovac, D.; Rattigan, S.; Richards, S.M.; Muniyappa, R.; Quon, M.J.; Keske, M.A. Acute vascular and metabolic actions of the green tea polyphenol epigallocatechin 3-gallate in rat skeletal muscle. J. Nutr. Biochem. 2017, 40, 23–31. [Google Scholar] [CrossRef]
- Yi, R.; Wang, R.; Sun, P.; Zhao, X. Antioxidant-mediated preventative effect of Dragon-pearl tea crude polyphenol extract on reserpine-induced gastric ulcers. Exp. Ther. Med. 2015, 10, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Kurata, S.; Sakurai, T. Simultaneous Analysis of Eight Catechins in Tea Drinks by Gas Chromatography/Mass Spectrometry. Bunseki Kagaku 2012, 61, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Fei, X.; Shen, Y.; Li, X.; Guo, H. The association of tea consumption and the risk and progression of prostate cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 3881–3891. [Google Scholar]
- Zhang, X.; Wu, Z.F.; Weng, P.F. Antioxidant and Hepatoprotective Effect of (-)-Epigallocatechin 3-O-(3-O-Methyl) gallate (EGCG3” Me) from Chinese Oolong Tea. J. Agric. Food Chem. 2014, 62, 10046–10054. [Google Scholar] [CrossRef]
- Astill, C.; Birch, M.R.; Dacombe, C.; Humphrey, P.G.; Martin, P.T. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J. Agric. Food Chem. 2001, 49, 5340–5347. [Google Scholar] [CrossRef]
- Robertson, A. Effects of Catechin Concentration on the Formation of Black Tea Polyphenols during Invitro Oxidation. Phytochemistry 1983, 22, 897–903. [Google Scholar] [CrossRef]
- Graham, H.N. Green Tea Composition, Consumption, and Polyphenol Chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Opie, S.C.; Clifford, M.N.; Robertson, A. The Formation of Thearubigin-Like Substances by in-Vitro Polyphenol Oxidase-Mediated Fermentation of Individual Flavan-3-Ols. J. Sci. Food Agric. 1995, 67, 501–505. [Google Scholar] [CrossRef]
- Kuhr, S.; Herzig, B.; Ehgelhardt, U.H. Studies of Polymer Polyphenols in Tea. Z. Lebensm Unters For. 1994, 199, 13–16. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Peterson, J. Tea and flavonoids: Where we are, where to go next. Am. J. Clin. Nutr. 2013, 98 (Suppl. S6), 1611s–1618s. [Google Scholar] [CrossRef] [Green Version]
- Pokorny, O. Antioxidativ Wirksame Verbindungen in Brombeerblättern, Cistus Incanus, Himbeerblättern, Pfefferminze und Anderen Kräuter- und Früchtetees; Technische Universität Braunschweig: Braunschweig, Germany, 2005. [Google Scholar]
- Hannig, C.; Spitzmuller, B.; Al-Ahmad, A.; Hannig, M. Effects of Cistus-tea on bacterial colonization and enzyme activities of the in situ pellicle. J. Dent. 2008, 36, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Wittpahl, G.; Kolling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, C. The Polyphenolic Composition of Cistus incanus Herbal Tea and Its Antibacterial and Anti-adherent Activity against Streptococcus mutans. Planta Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petereit, F.; Kolodziej, H.; Nahrstedt, A. Flavan-3-Ols and Proanthocyanidins from Cistus-Incanus. Phytochemistry 1991, 30, 981–985. [Google Scholar] [CrossRef]
- Petereit, F.; Kolodziej, H.; Nahrstedt, A. Proanthocyanidins and Biogenetically Related Dihydroflavonols from Cistus-Incanus L. Basic Life Sci. 1992, 59, 729–737. [Google Scholar]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Roldan, C.; Guillen, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A Systematic Study of the Polyphenolic Composition of Aqueous Extracts Deriving from Several Cistus Genus Species: Evolutionary Relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Pomponio, R.; Gotti, R.; Santagati, N.A.; Cavrini, V. Analysis of catechins in extracts of Cistus species by microemulsion electrokinetic chromatography. J. Chromatogr. A 2003, 990, 215–223. [Google Scholar] [CrossRef]
- Rehage, M.; Delius, J.; Hofmann, T.; Hannig, M. Oral astringent stimuli alter the enamel pellicle’s ultrastructure as revealed by electron microscopy. J. Dent. 2017, 63, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, H.; Hosni, K.; Zaouali, W.; Amri, I.; Zargouni, H.; Hamida, N.B.; Kaddour, R.; Hamrouni, L.; Nasri, M.B.; Ouerghi, Z. Comprehensive Phytochemical Analysis, Antioxidant and Antifungal Activities of Inula viscosa Aiton Leaves. J. Food Saf. 2016, 36, 77–88. [Google Scholar] [CrossRef]
- Gonzali, S.; Perata, P. Anthocyanins from Purple Tomatoes as Novel Antioxidants to Promote Human Health. Antioxidants 2020, 9, 1017. [Google Scholar] [CrossRef]
- Talib, W.H.; Abu Zarga, M.H.; Mahasneh, A.M. Antiproliferative, Antimicrobial and Apoptosis Inducing Effects of Compounds Isolated from Inula viscosa. Molecules 2012, 17, 3291–3303. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.N.; Lee, Y.J.; Choi, J.H.; Jin, M.; Yang, J.H.; Li, Y.; Lee, J.; Li, X.; Kim, K.J.; Son, J.K.; et al. Alleviation of OVA-Induced Airway Inflammation by Flowers of Inula japonica in a Murine Model of Asthma. Biosci. Biotechnol. Biochem. 2011, 75, 871–876. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Pridgeon, J.W.; Fronczek, F.R.; Becnel, J.J. Structure—Activity Relationship Studies on Derivatives of Eudesmanolides from Inula helenium as Toxicants against Aedes aegypti Larvae and Adults. Chem. Biodivers. 2010, 7, 1681–1697. [Google Scholar] [CrossRef]
- Brahmi-Chendouh, N.; Piccolella, S.; Crescente, G.; Pacifico, F.; Boulekbache, L.; Hamri-Zeghichi, S.; Akkal, S.; Madani, K.; Pacifico, S. A nutraceutical extract from Inula viscosa leaves: UHPLC-HR-MS/MS based polyphenol profile, and antioxidant and cytotoxic activities. J. Food Drug Anal. 2019, 27, 692–702. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Grande, M.; Piera, F.; Cuenca, A.; Torres, P.; Bellido, I.S. Flavonoids from Inula-Viscosa. Planta Med. 1985, 51, 414–419. [Google Scholar] [CrossRef]
- Wollenweber, E.; Mayer, K.; Roitman, J.N. Exudate Flavonoids of Inula-Viscosa. Phytochemistry 1991, 30, 2445–2446. [Google Scholar] [CrossRef]
- Hertel, S.; Graffy, L.; Potschke, S.; Basche, S.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Effect of Inula viscosa on the pellicle’s protective properties and initial bioadhesion in-situ. Arch. Oral Biol. 2016, 71, 87–96. [Google Scholar] [CrossRef]
- Najda, A.; Dyduch, J.; Dyduch-Siemińska, M.; Gantner, M. Comparative analysis of secondary metabolites contents in Fragaria vesca L. fruits. Ann. Agric. Environ. Med. 2014, 21, 339–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichtl, M. Herbal Drugs and Phytopharmaceuticals: A Handbook for Practice on a Scientific Basis, 3rd ed.; Medpharm: Stuttgart, Germany; CRC Press: Boca Raton, FL, USA, 2004; 704p. [Google Scholar]
- D’Urso, G.; Maldini, M.; Pintore, G.; d’Aquino, L.; Montoro, P.; Pizza, C. Characterisation of Fragaria vesca fruit from Italy following a metabolomics approach through integrated mass spectrometry techniques. LWT-Food Sci. Technol. 2016, 74, 387–395. [Google Scholar] [CrossRef]
- Mudnic, I.; Modun, D.; Brizic, I.; Vukovic, J.; Generalic, I.; Katalinic, V.; Bilusic, T.; Ljubenkov, I.; Boban, M. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca L.) leaves. Phytomedicine 2009, 16, 462–469. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profile and antioxidant properties of commercial and wild Fragaria vesca L. roots: A comparison between hydromethanolic and aqueous extracts. Ind. Crop. Prod. 2015, 63, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girao, H.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Fragaria vesca leaves through inflammation, proteasome and autophagy modulation. J. Ethnopharmacol. 2014, 158, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Vennat, B.; Pourrat, H.; Pouget, M.P.; Gross, D.; Pourrat, A. Tannins from Hamamelis-Virginiana—Identification of Proanthocyanidins and Hamamelitannin Quantification in Leaf, Bark, and Stem Extracts. Planta Med. 1988, 54, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.J.; Torres, J.L.; Medina, I. Impact of Thermal Processing on the Activity of Gallotannins and Condensed Tannins from Hamamelis virginiana Used as Functional Ingredients in Seafood. J. Agric. Food Chem. 2010, 58, 4274–4283. [Google Scholar] [CrossRef]
- Tomczyk, M.; Latté, K.P. Potentilla—A review of its phytochemical and pharmacological profile. J Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; Casetti, F.; Bullerkotte, U.; Haarhaus, B.; Vagedes, J.; Schempp, C.M.; Wolfle, U. Anti-Inflammatory Effects of Agrimoniin-Enriched Fractions of Potentilla erecta. Molecules 2016, 21, 792. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Fecka, I.; Weglinska, M.; Szostak, R. Quantification of active ingredients in Potentilla tormentilla by Raman and infrared spectroscopy. Talanta 2018, 189, 308–314. [Google Scholar] [CrossRef]
- Mari, A.; Eletto, D.; Pizza, C.; Montoro, P.; Piacente, S. Integrated mass spectrometry approach to profile proanthocyanidins occurring in food supplements: Analysis of Potentilla erecta L. rhizomes. Food Chem. 2013, 141, 4171–4178. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Kuhnert, N. Polyphenole: Vielseitige Pflanzeninhaltsstoffe. Chem. Unserer Zeit 2013, 47, 80–91. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and Metabolism of Phenolic Compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley: Hoboken, NJ, USA, 2018; Volumes I & II, pp. 115–123. [Google Scholar]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, K.M. The Shikimate Pathway as an Entry to Aromatic Secondary Metabolism. Plant Physiol. 1995, 107, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, K.M. The Shikimate Pathway—Early Steps in the Biosynthesis of Aromatic-Compounds. Plant Cell 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Kurosaki, F.; Itoh, M.; Nishi, A. Interaction between Cerulenin and 6-Hydroxymellein Synthase in Carrot Cell-Extracts. Phytochemistry 1994, 35, 297–299. [Google Scholar] [CrossRef]
- Whetten, R.; Sederoff, R. Lignin Biosynthesis. Plant Cell 1995, 7, 1001–1013. [Google Scholar] [CrossRef]
- Dewick, P.M.; Haslam, E. Phenol Biosynthesis in Higher Plants—Gallic Acids. Biochem. J. 1969, 113, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenk, M.H. Zur Frage Der Biosynthese Von Gallussaure. Z. Naturforsch. B 1964, 19, 83. [Google Scholar] [CrossRef]
- Saijo, R. Pathway of Gallic Acid Biosynthesis and Its Esterification with Catechins in Young Tea Shoots. Agric. Biol. Chem. Tokyo 1983, 47, 455–460. [Google Scholar] [CrossRef]
- Hofmann, A.S.; Gross, G.G. Biosynthesis of gallotannins: Formation of polygalloylglucoses by enzymatic acylation of 1,2,3,4,6-penta-O-galloylglucose. Arch. Biochem. Biophys. 1990, 283, 530–532. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Dubrovina, A.S.; Kiselev, K.V. Regulation of stilbene biosynthesis in plants. Planta 2017, 246, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Riceevans, C. Polyphenolic Flavanols as Scavengers of Aqueous-Phase Radicals and as Chain-Breaking Antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Liu, Q.; Mao, Q.Q.; Shang, A.; Li, H.B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.F. Research progress on theaflavins: Efficacy, formation, and preparation. Food Nutr. Res. 2017, 61, 1344521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosuge, T.; Conn, E.E. The metabolism of aromatic compounds in higher plants. I. Coumarin and o-coumaric acid. J. Biol. Chem. 1959, 234, 2133–2137. [Google Scholar] [CrossRef]
- Zhu, J.J.; Jiang, J.G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure-Activity Relationships. Mol. Nutr. Food Res. 2018, 62, 1701073. [Google Scholar] [CrossRef] [PubMed]
- Morel, I.; Lescoat, G.; Cogrel, P.; Sergent, O.; Pasdeloup, N.; Brissot, P.; Cillard, P.; Cillard, J. Antioxidant and Iron-Chelating Activities of the Flavonoids Catechin, Quercetin and Diosmetin on Iron-Loaded Rat Hepatocyte Cultures. Biochem. Pharmacol. 1993, 45, 13–19. [Google Scholar] [CrossRef]
- Foti, M.; Piattelli, M.; Baratta, M.T.; Ruberto, G. Flavonoids, coumarins, and cinnamic acids as antioxidants in a micellar system. Structure-activity relationship. J. Agric. Food Chem. 1996, 44, 497–501. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; van Trijp, J.M.P.; Mengelers, M.J.B.; de Vries, J.H.M.; Katan, M.B. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997, 114, 139–140. [Google Scholar] [CrossRef]
- Ader, P.; Grenacher, B.; Langguth, P.; Scharrer, E.; Wolffram, S. Cinnamate uptake by rat small intestine: Transport kinetics and transepithelial transfer. Exp. Physiol. 1996, 81, 943–955. [Google Scholar] [CrossRef] [Green Version]
- Vanholme, R.; De Meester, B.; Ralph, J.; Boerjan, W. Lignin biosynthesis and its integration into metabolism. Curr. Opin. Biotechnol. 2019, 56, 230–239. [Google Scholar] [CrossRef]
- Cuadra, P.; Harborne, J.B.; Waterman, P.G. Increases in surface flavonols and photosynthetic pigments in Gnaphalium luteo-album in response to UV-B radiation. Phytochemistry 1997, 45, 1377–1383. [Google Scholar] [CrossRef]
- Olsson, L.C.; Veit, M.; Weissenbock, G.; Bornman, J.F. Differential flavonoid response to enhanced UV-B radiation in Brassica napus. Phytochemistry 1998, 49, 1021–1028. [Google Scholar] [CrossRef]
- Majer, P.; Neugart, S.; Krumbein, A.; Schreiner, M.; Hideg, E. Singlet oxygen scavenging by leaf flavonoids contributes to sunlight acclimation in Tilia platyphyllos. Environ. Exp. Bot. 2014, 100, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zito, P.; Rosselli, S.; Bruno, M.; Maggio, A.; Sajeva, M. Floral scent in Iris planifolia (Iridaceae) suggests food reward. Phytochemistry 2019, 158, 86–90. [Google Scholar] [CrossRef]
- Soares, S.; Brandao, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef]
- Appendino, G.; Szallasi, A. Euphorbium: Modern research on its active principle, resiniferatoxin, revives an ancient medicine. Life Sci. 1997, 60, 681–696. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. The Pellicle and Erosion. Erosive Tooth Wear: From Diagnosis to Therapy, 2nd ed.; Karger: Berlin, Germany, 2014; Volume 25, pp. 206–214. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. The oral cavity—a key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Proctor, G.B.; Yakubov, G.E.; Wilson, S.; Carpenter, G.H. Concentration of salivary protective proteins within the bound oral mucosal pellicle. Oral Dis. 2014, 20, 707–713. [Google Scholar] [CrossRef] [Green Version]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What interactions drive the salivary mucosal pellicle formation? Colloid Surf. B 2014, 120, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Gibbins, H.L.; Proctor, G.B.; Yakubov, G.E.; Wilson, S.; Carpenter, G.H. SIgA binding to mucosal surfaces is mediated by mucin-mucin interactions. PLoS ONE 2015, 10, e0119677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradway, S.D.; Bergey, E.J.; Jones, P.C.; Levine, M.J. Oral Mucosal Pellicle—Adsorption and Transpeptidation of Salivary Components to Buccal Epithelial-Cells. Biochem. J. 1989, 261, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Bradway, S.D.; Bergey, E.J.; Scannapieco, F.A.; Ramasubbu, N.; Zawacki, S.; Levine, M.J. Formation of Salivary-Mucosal Pellicle—the Role of Transglutaminase. Biochem. J. 1992, 284, 557–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitkov, L.; Krautgartner, W.D.; Hannig, M.; Fuchs, K. Fimbria-mediated bacterial adhesion to human oral epithelium. FEMS Microbiol. Lett. 2001, 202, 25–30. [Google Scholar] [CrossRef]
- Vitkov, L.; Krautgartner, W.D.; Hannig, M.; Weitgasser, R.; Stoiber, W. Candida attachment to oral epithelium. Oral Microbiol. Immun. 2002, 17, 60–64. [Google Scholar] [CrossRef]
- Vitkov, L.; Hannig, M.; Krautgartner, W.D.; Fuchs, K. Bacterial attachment to gingiva in periodontitis. J. Dent. Res. 2002, 81, B287. [Google Scholar]
- Chagnot, C.; Zorgani, M.A.; Astruc, T.; Desvaux, M. Proteinaceous determinants of surface colonization in bacteria: Bacterial adhesion and biofilm formation from a protein secretion perspective. Front. Microbiol. 2013, 4, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asikainen, P.; Ruotsalainen, T.J.; Mikkonen, J.J.W.; Koistinen, A.; ten Bruggenkate, C.; Kullaa, A.M. The defence architecture of the superficial cells of the oral mucosa. Med. Hypotheses 2012, 78, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Morzel, M.; Siying, T.; Brignot, H.; Lherminier, J. Immunocytological detection of salivary mucins (MUC5B) on the mucosal pellicle lining human epithelial buccal cells. Microsc. Res. Tech. 2014, 77, 453–457. [Google Scholar] [CrossRef]
- Kullaa, A.M.; Asikainen, P.; Herrala, M.; Ukkonen, H.; Mikkonen, J.J.W. Microstructure of Oral Epithelial Cells as an Underlying Basis for Salivary Mucosal Pellicle. Ultrastruct Pathol. 2014, 38, 382–386. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Belloir, C.; Bonnotte, A.; Bourillot, E.; Briand, L.; Lesniewska, E.; Lherminier, J.; Aybeke, E.; Canon, F. Mechanisms of astringency: Structural alteration of the oral mucosal pellicle by dietary tannins and protective effect of bPRPs. Food Chem. 2018, 253, 79–87. [Google Scholar] [CrossRef]
- Siqueira, W.L.; Custodio, W.; McDonald, E.E. New Insights into the Composition and Functions of the Acquired Enamel Pellicle. J. Dent. Res. 2012, 91, 1110–1118. [Google Scholar] [CrossRef]
- Lendenmann, U.; Grogan, J.; Oppenheim, F.G. Saliva and dental pellicle—A review. Adv. Dent. Res. 2000, 14, 22–28. [Google Scholar] [CrossRef]
- Trautmann, S.; Barghash, A.; Fecher-Trost, C.; Schalkowsky, P.; Hannig, C.; Kirsch, J.; Rupf, S.; Keller, A.; Helms, V.; Hannig, M. Proteomic Analysis of the Initial Oral Pellicle in Caries-Active and Caries-Free Individuals. Proteom. Clin. Appl. 2019, 13, e1800143. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Joiner, A. The structure, function and properties of the acquired pellicle. Monogr. Oral Sci. 2006, 19, 29–64. [Google Scholar] [CrossRef]
- Leach, S.A.; Critchley, P.; Kolendo, A.B.; Saxton, C.A. Salivary glycoproteins as components of the enamel integuments. Caries Res. 1967, 1, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Guth-Thiel, S.; Kraus-Kuleszka, I.; Mantz, H.; Hoth-Hannig, W.; Hahl, H.; Dudek, J.; Jacobs, K.; Hannig, M. Comprehensive measurements of salivary pellicle thickness formed at different intraoral sites on Si wafers and bovine enamel. Colloids Surf. B Biointerfaces 2019, 174, 246–251. [Google Scholar] [CrossRef]
- Hannig, M. Ultrastructural investigation of pellicle morphogenesis at two different intraoral sites during a 24-h period. Clin. Oral Investig. 1999, 3, 88–95. [Google Scholar] [CrossRef]
- Adams, D.; Addy, M. Mouthrinses. Adv. Dent. Res. 1994, 8, 291–301. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Higham, S.M.; Edgar, W.M.; Milosevic, A. Thickness of acquired salivary pellicle as a determinant of the sites of dental erosion. J. Dent. Res. 1999, 78, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Bennick, A.; Cannon, M.; Madapallimattam, G. The nature of the hydroxyapatite-binding site in salivary acidic proline-rich proteins. Biochem. J. 1979, 183, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannig, M.; Balz, M. Influence of in vivo formed salivary pellicle on enamel erosion. Caries Res. 1999, 33, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, J.; Jung, A.; Hille, K.; König, B.; Hannig, C.; Kölling-Speer, I.; Speer, K.; Hannig, M. Effect of fragaria vesca, hamamelis and tormentil on the initial bacterial colonization in situ. Arch. Oral Biol. 2020, 118, 104853. [Google Scholar] [CrossRef] [PubMed]
- Delius, J.; Trautmann, S.; Medard, G.; Kuster, B.; Hannig, M.; Hofmann, T. Label-free quantitative proteome analysis of the surface-bound salivary pellicle. Colloids Surf. B Biointerfaces 2017, 152, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, S.; Kunzel, N.; Fecher-Trost, C.; Barghash, A.; Schalkowsky, P.; Dudek, J.; Delius, J.; Helms, V.; Hannig, M. Deep Proteomic Insights into the Individual Short-Term Pellicle Formation on Enamel-An In Situ Pilot Study. Proteom. Clin. Appl. 2020, 14, e2070054. [Google Scholar] [CrossRef]
- Douglas, W.H.; Reeh, E.S.; Ramasubbu, N.; Raj, P.A.; Bhandary, K.K.; Levine, M.J. Statherin—A Major Boundary Lubricant of Human Saliva. Biochem. Biophys. Res. Commun. 1991, 180, 91–97. [Google Scholar] [CrossRef]
- Zahradnik, R.T.; Moreno, E.C.; Burke, E.J. Effect of Salivary Pellicle on Enamel Subsurface Demineralization Invitro. J. Dent. Res. 1976, 55, 664–670. [Google Scholar] [CrossRef]
- Laible, N.J.; Germaine, G.R. Bactericidal Activity of Human Lysozyme, Muramidase-Inactive Lysozyme, and Cationic Polypeptides against Streptococcus-Sanguis and Streptococcus-Faecalis—Inhibition by Chitin Oligosaccharides. Infect. Immun. 1985, 48, 720–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira, W.L.; Zhang, W.M.; Helmerhorst, E.J.; Gygi, S.P.; Oppenheim, F.G. Identification of protein components in in vivo human acquired enamel pellicle using LC-ESI-MS/MS. J. Proteome Res. 2007, 6, 2152–2160. [Google Scholar] [CrossRef]
- Yao, Y.; Grogan, J.; Zehnder, M.; Lendenmann, U.; Nam, B.; Wu, Z.; Costello, C.E.; Oppenheim, F.G. Compositional analysis of human acquired enamel pellicle by mass spectrometry. Arch. Oral Biol. 2001, 46, 293–303. [Google Scholar] [CrossRef]
- Nobbs, A.H.; Jenkinson, H.F.; Jakubovics, N.S. Stick to Your Gums: Mechanisms of Oral Microbial Adherence. J. Dent. Res. 2011, 90, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Ruhl, S.; Sandberg, A.L.; Cisar, J.O. Salivary receptors for the proline-rich protein-binding and lectin-like adhesins of oral actinomyces and streptococci. J. Dent. Res. 2004, 83, 505–510. [Google Scholar] [CrossRef]
- Cisar, J.O.; Sandberg, A.L.; Reddy, G.P.; Abeygunawardana, C.; Bush, C.A. Structural and antigenic types of cell wall polysaccharides from viridans group streptococci with receptors for oral actinomyces and streptococcal lectins. Infect. Immun. 1997, 65, 5035–5041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannig, C.; Kirsch, J.; Al-Ahmad, A.; Kensche, A.; Hannig, M.; Kummerer, K. Do edible oils reduce bacterial colonization of enamel in situ? Clin. Oral Investig. 2013, 17, 649–658. [Google Scholar] [CrossRef]
- Kensche, A.; Reich, M.; Kummerer, K.; Hannig, M.; Hannig, C. Lipids in preventive dentistry. Clin. Oral Investig. 2013, 17, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar] [CrossRef]
- Liljemark, W.F.; Bloomquist, C. Human oral microbial ecology and dental caries and periodontal diseases. Crit. Rev. Oral Biol. Med. 1996, 7, 180–198. [Google Scholar] [CrossRef]
- Marsh, P.D.; Martin, M.V. Orale Mikrobiologie; Georg Thieme Verlag: Stuttgart, Germany; Thieme Publishers: New York, NY, USA, 2003. [Google Scholar]
- Quirynen, M.; Bollen, C.M. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J. Clin. Periodontol. 1995, 22, 1–14. [Google Scholar] [CrossRef]
- Loesche, W.J. Role of Streptococcus-Mutans in Human Dental Decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Streptococcus Adherence and Colonization. Microbiol. Mol. Biol. Rev. 2009, 73, 407–450. [Google Scholar] [CrossRef] [Green Version]
- Gregoire, S.; Xiao, J.; Silva, B.B.; Gonzalez, I.; Agidi, P.S.; Klein, M.I.; Ambatipudi, K.S.; Rosalen, P.L.; Bauserman, R.; Waugh, R.E.; et al. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 2011, 77, 6357–6367. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Koo, H.; Falsetta, M.L.; Klein, M.I. The Exopolysaccharide Matrix: A Virulence Determinant of Cariogenic Biofilm. J. Dent. Res. 2013, 92, 1065–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, B.I.; Bae, J.Y.; An, Y.K.; Jung, D.H.; Seo, D.H.; Jung, D.H. Construction of glucans-polymerized enzyme system (G-PES) and its application to functional sugar biosynthesis. New Biotechnol. 2016, 33, S200. [Google Scholar] [CrossRef]
- Guo, L.H.; McLean, J.S.; Lux, R.; He, X.S.; Shi, W.Y. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci. Rep. 2015, 5, 18015. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.I.; Debaz, L.; Agidi, S.; Lee, H.; Xie, G.; Lin, A.H.M.; Hamaker, B.R.; Lemos, J.A.; Koo, H. Dynamics of Streptococcus mutans Transcriptome in Response to Starch and Sucrose during Biofilm Development. PLoS ONE. 2010, 5, e13478. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.I.; Duarte, S.; Xiao, J.; Mitra, S.; Foster, T.H.; Koo, H. Structural and Molecular Basis of the Role of Starch and Sucrose in Streptococcus mutans Biofilm Development. Appl. Environ. Microb. 2009, 75, 837–841. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.C.C.; Tabchoury, C.P.M.; Del Bel Cury, A.A.; Tenuta, L.M.A.; Rosalen, P.L.; Cury, J.A. Effect of starch on the cariogenic potential of sucrose. Brit. J. Nutr. 2005, 94, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Hertel, S.; Potschke, S.; Basche, S.; Delius, J.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Effect of Tannic Acid on the Protective Properties of the in situ Formed Pellicle. Caries Res. 2017, 51, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.T.; Hannig, M.; Potschke, S.; Hohne, F.; Hannig, C. Application of Plant Extracts for the Prevention of Dental Erosion: An in situ/in vitro Study. Caries Res. 2015, 49, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Joiner, A.; Muller, D.; Elofsson, U.M.; Arnebrant, T. Ellipsometry analysis of the in vitro adsorption of tea polyphenols onto salivary pellicles. Eur. J. Oral Sci. 2004, 112, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Joiner, A.; Muller, D.; Elofsson, U.M.; Malmsten, M.; Arnebrant, T. Adsorption from black tea and red wine onto in vitro salivary pellicles studied by ellipsometry. Eur. J. Oral Sci. 2003, 111, 417–422. [Google Scholar] [CrossRef]
- Zimmermann, R.; Delius, J.; Friedrichs, J.; Stehl, S.; Hofmann, T.; Hannig, C.; Rehage, M.; Werner, C.; Hannig, M. Impact of oral astringent stimuli on surface charge and morphology of the protein-rich pellicle at the tooth-saliva interphase. Colloids Surf. B Biointerfaces 2019, 174, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Sorg, J.; Spitzmuller, B.; Hannig, M.; Al-Ahmad, A. Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J. Dent. 2009, 37, 560–566. [Google Scholar] [CrossRef]
- Niemeyer, S.H.; Baumann, T.; Lussi, A.; Meyer-Lueckel, H.; Scaramucci, T.; Carvalho, T.S. Salivary pellicle modification with polyphenol-rich teas and natural extracts to improve protection against dental erosion. J Dent. 2021, 105, 103567. [Google Scholar] [CrossRef]
- Nayak, A.; Carpenter, G.H. A physiological model of tea-induced astringency. Physiol. Behav. 2008, 95, 290–294. [Google Scholar] [CrossRef]
- Yan, Q.Y.; Bennick, A. Identification of Histatins as Tannin-Binding Proteins in Human Saliva. Biochem. J. 1995, 311, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, N.J.; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochemistry 1997, 36, 5566–5577. [Google Scholar] [CrossRef] [PubMed]
- Jobstl, E.; O’Connell, J.; Fairclough, J.P.A.; Williamson, M.P. Molecular model for astringency produced by polyphenol/protein interactions. Biomacromolecules 2004, 5, 942–949. [Google Scholar] [CrossRef]
- De Souza, E.S.C.M.; da Silva Ventura, T.M.; de Pau, L.; la Silva, C.; de Lima Leite, A.; Buzalaf, M.A.R. Effect of gels containing chlorhexidine or epigallocatechin-3-gallate on the protein composition of the acquired enamel pellicle. Arch. Oral Biol. 2017, 82, 92–98. [Google Scholar] [CrossRef]
- Ares, G.; Barreiro, C.; Deliza, R.; Gambaro, A. Alternatives to reduce the bitterness, astringency and characteristic flavour of antioxidant extracts. Food Res. Int. 2009, 42, 871–878. [Google Scholar] [CrossRef]
- Schupbach, P.; Oppenheim, F.G.; Lendenmann, U.; Lamkin, M.S.; Yao, Y.; Guggenheim, B. Electron-microscopic demonstration of proline-rich proteins, statherin, and histatins in acquired enamel pellicles in vitro. Eur. J. Oral Sci. 2001, 109, 60–68. [Google Scholar] [CrossRef]
- Bennick, A. Salivary proline-rich proteins. Mol. Cell. Biochem. 1982, 45, 83–99. [Google Scholar] [CrossRef]
- Frenkel, E.S.; Ribbeck, K. Salivary Mucins Protect Surfaces from Colonization by Cariogenic Bacteria. Appl. Environ. Microb. 2015, 81, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlesinger, D.H.; Hay, D.I. Complete Covalent Structure of Statherin, a Tyrosine-Rich Acidic Peptide Which Inhibits Calcium-Phosphate Precipitation from Human Parotid Saliva. J. Biol. Chem. 1977, 252, 1689–1695. [Google Scholar] [CrossRef]
- Yao, J.W.; Lin, C.J.; Chen, G.Y.; Lin, F.; Tao, T. The interactions of epigallocatechin-3-gallate with human whole saliva and parotid saliva. Arch. Oral Biol. 2010, 55, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Jean-Gilles, D.; Li, L.Y.; Vaidyanathan, V.G.; King, R.; Cho, B.; Worthen, D.R.; Chichester, C.O.; Seeram, N.P. Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem.-Biol. Interact. 2013, 205, 90–99. [Google Scholar] [CrossRef]
- Kandra, L.; Gyemant, G.; Zajacz, A.; Batta, G. Inhibitory effects of tannin on human salivary alpha-amylase. Biochem. Biophys. Res. Commun. 2004, 319, 1265–1271. [Google Scholar] [CrossRef]
- Yanagida, A.; Kanda, T.; Tanabe, M.; Matsudaira, F.; Cordeiro, J.G.O. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J. Agric. Food Chem. 2000, 48, 5666–5671. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Spitzmuller, B.; Hoth-Hannig, W.; Hannig, M. Targeted immobilisation of lysozyme in the enamel pellicle from different solutions. Clin. Oral Investig. 2011, 15, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Lambert, J.D.; Prabhu, S.; Meng, X.; Lu, H.; Maliakal, P.; Ho, C.T.; Yang, C.S. Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extract. Cancer Epidemiol. Biomark. Prev. 2004, 13, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Hannig, C.; Spitzmuller, B.; Hannig, M. Characterisation of lysozyme activity in the in situ pellicle using a fluorimetric assay. Clin. Oral Investig. 2009, 13, 15–21. [Google Scholar] [CrossRef]
- Abdollahzadeh, S.; Mashouf, R.; Mortazavi, H.; Moghaddam, M.; Roozbahani, N.; Vahedi, M. Antibacterial and antifungal activities of Punica granatum peel extracts against oral pathogens. J. Dent. 2011, 8, 1–6. [Google Scholar]
- Naz, S.; Siddiqi, R.; Ahmad, S.; Rasool, S.; Sayeed, S.A. Antibacterial activity directed isolation of compounds from Punica granatum. J. Food Sci. 2007, 72, M341–M345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.M.; Visser, R.G.F.; Bovy, A. Anthocyanin Biosynthesis and Degradation Mechanisms in Solanaceous Vegetables: A Review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073s–2085s. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional compounds from olive pomace to obtain high-added value foods—A review. J. Sci. Food Agric. 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Wightman, J.D. Potential health benefits of (poly)phenols derived from fruit and 100% fruit juice. Nutr. Rev. 2020, 78, 145–174. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Polyviou, T.; Ludwig, I.A.; Nastase, A.M.; Moreno-Rojas, J.M.; Garcia, A.L.; Malkova, D.; Crozier, A. Bioavailability of orange juice (poly) phenols: The impact of short-term cessation of training by male endurance athletes. Am. J. Clin. Nutr. 2017, 106, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant Potential Overviews of Secondary Metabolites (Polyphenols) in Fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Fourati, M.; Smaoui, S.; Ben Hlima, H.; Elhadef, K.; Ben Braiek, O.; Ennouri, K.; Mtibaa, A.C.; Mellouli, L. Bioactive Compounds and Pharmacological Potential of Pomegranate (Punica granatum) Seeds—A Review. Plant Food Hum. Nutr. 2020, 75, 477–486. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T. Anti-cariogenic properties of tea (Camellia sinensis). J. Med. Microbiol. 2001, 50, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Otake, S.; Makimura, M.; Kuroki, T.; Nishihara, Y.; Hirasawa, M. Anticaries Effects of Polyphenolic Compounds from Japanese Green Tea. Caries Res. 1991, 25, 438–443. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ogawa, T. Antimicrobial activity of perilla seed polyphenols against oral pathogenic bacteria. Biosci. Biotechnol. Biochem. 2002, 66, 921–924. [Google Scholar] [CrossRef]
- Osawa, K.; Yasuda, H.; Maruyama, T.; Morita, H.; Takeya, K.; Itokawa, H. Isoflavanones from the Heartwood of Swartzia-Polyphylla and Their Antibacterial Activity against Cariogenic Bacteria. Chem. Pharm. Bull. 1992, 40, 2970–2974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, H.; Schobel, B.; Scott-Anne, K.; Watson, G.; Bowen, W.H.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Marquis, R.E.; Bowen, W.H. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 2005, 84, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Pearson, S.K.; Scott-Anne, K.; Abranches, J.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Marquis, R.E.; Bowen, W.H. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol. Immun. 2002, 17, 337–343. [Google Scholar] [CrossRef]
- Nasser, M.; Housheh, S.; Kourini, A.; Maala, N. Chemical Composition of Essential Oil from Leaves and Flowers of Inula Viscosa (L.) in Al-Qadmous Region, Syria. Int. J. Pharm. Sci. Res. 2014, 5, 5177–5182. [Google Scholar] [CrossRef]
- Takahashi, N.; Yamada, T. Acid-induced acidogenicity and acid tolerance of non-mutans streptococci. J. Dent. Res. 1998, 77, 286. [Google Scholar] [CrossRef]
- Schilling, K.M.; Bowen, W.H. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect. Immun. 1992, 60, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Hannig, C.; Hannig, M.; Attin, T. Enzymes in the acquired enamel pellicle. Eur. J. Oral Sci. 2005, 113, 2–13. [Google Scholar] [CrossRef]
- Deimling, D.; Breschi, L.; Hoth-Hannig, W.; Ruggeri, A.; Hannig, C.; Nekrashevych, Y.; Prati, C.; Hannig, M. Electron microscopic detection of salivary alpha-amylase in the pellicle formed in situ. Eur. J. Oral Sci. 2004, 112, 503–509. [Google Scholar] [CrossRef]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary Metabolites, Anthocyanins, and Hydrolyzable Tannins in the Pomegranate Fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landazuri, P.; Loango, N.; Aguillon, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksic, M.F.; Zagorac, D.D.; Sredojevic, M.; Milivojevic, J.; Gasic, U.; Meland, M.; Natic, M. Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System. Molecules 2019, 24, 4310. [Google Scholar] [CrossRef] [Green Version]
- Lamuel-Raventos, R.M.; Onge, M.S. Prebiotic nut compounds and human microbiota. Crit. Rev. Food Sci. Nutr. 2017, 57, 3154–3163. [Google Scholar] [CrossRef]
- Bolling, B.W.; Chen, C.Y.; McKay, D.L.; Blumberg, J.B. Tree nut phytochemicals: Composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr. Res. Rev. 2011, 24, 244–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavez-Mendoza, C.; Sanchez, E. Bioactive Compounds from Mexican Varieties of the Common Bean (Phaseolus vulgaris): Implications for Health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef] [Green Version]
- Hithamani, G.; Srinivasan, K. Bioaccessibility of polyphenols from selected cereal grains and legumes as influenced by food acidulants. J. Sci. Food Agric. 2017, 97, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.R.; Delgado, A.M.; Issaoui, M.; Chammem, N.; Fiorino, M.; Pellerito, A.; Natalello, S. Review of the Role of Fluid Dairy in Delivery of Polyphenolic Compounds in the Diet: Chocolate Milk, Coffee Beverages, Matcha Green Tea, and Beyond. J. AOAC Int. 2019, 102, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C.M. The chemistry of tea flavonoids. Crit. Rev. Food Sci. 1997, 37, 693–704. [Google Scholar] [CrossRef]
- Drynan, J.W.; Clifford, M.N.; Obuchowicz, J.; Kuhnert, N. The chemistry of low molecular weight black tea polyphenols. Nat. Prod. Rep. 2010, 27, 417–462. [Google Scholar] [CrossRef]
- Wang, Y.; Ho, C.T. Polyphenolic Chemistry of Tea and Coffee: A Century of Progress. J. Agric. Food Chem. 2009, 57, 8109–8114. [Google Scholar] [CrossRef]
- Hannig, C.; Spitzmuller, B.; Knausenberger, S.; Hoth-Hannig, W.; Hellwig, E.; Hannig, M. Detection and activity of peroxidase in the in situ formed enamel pellicle. Arch. Oral Biol. 2008, 53, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.M.; Bowen, W.H. The activity of glucosyltransferase adsorbed onto saliva-coated hydroxyapatite. J. Dent. Res. 1988, 67, 2–8. [Google Scholar] [CrossRef]
- Vacca Smith, A.M.; Bowen, W.H. In situ studies of pellicle formation on hydroxyapatite discs. Arch. Oral Biol. 2000, 45, 277–291. [Google Scholar] [CrossRef]
- Dinnella, C.; Recchia, A.; Fia, G.; Bertuccioli, M.; Monteleone, E. Saliva Characteristics and Individual Sensitivity to Phenolic Astringent Stimuli. Chem. Senses 2009, 34, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Charlton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P. Polyphenol/peptide binding and precipitation. J. Agric. Food Chem. 2002, 50, 1593–1601. [Google Scholar] [CrossRef]
- Siebert, K.J.; Troukhanova, N.V.; Lynn, P.Y. Nature of polyphenol-protein interactions. J. Agric. Food Chem. 1996, 44, 80–85. [Google Scholar] [CrossRef]
- Soares, S.; Mateus, N.; de Freitas, V. Carbohydrates inhibit salivary proteins precipitation by condensed tannins. J. Agric. Food Chem. 2012, 60, 3966–3972. [Google Scholar] [CrossRef]
- Jobstl, E.; Howse, J.R.; Fairclough, J.P.A.; Williamson, M.P. Noncovalent cross-linking of casein by epigallocatechin gallate characterized by single molecule force microscopy. J. Agric. Food Chem. 2006, 54, 4077–4081. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.I.; Hoff, J.E.; Armstrong, G.S.; Haff, L.A. Hydrophobic Interaction in Tannin-Protein Complexes. J. Agric. Food Chem. 1980, 28, 394–398. [Google Scholar] [CrossRef]
- Kalyanaraman, B.; Premovic, P.I.; Sealy, R.C. Semiquinone Anion Radicals from Addition of Amino-Acids, Peptides, and Proteins to Quinones Derived from Oxidation of Catechols and Catecholamines—An Electron-Spin-Resonance Spin Stabilization Study. J. Biol. Chem. 1987, 262, 11080–11087. [Google Scholar] [CrossRef]
- Fontoin, H.; Saucier, C.; Teissedre, P.L.; Glories, Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Prefer. 2008, 19, 286–291. [Google Scholar] [CrossRef]
- Kawamoto, H.; Nakatsubo, F. Effects of environmental factors on two-stage tannin-protein co-precipitation. Phytochemistry 1997, 46, 479–483. [Google Scholar] [CrossRef]
- Mcrae, J.M.; Kennedy, J.A. Wine and Grape Tannin Interactions with Salivary Proteins and Their Impact on Astringency: A Review of Current Research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajec, M.R.; Pickering, G.J. Astringency: Mechanisms and perception. Crit. Rev. Food Sci. 2008, 48, 858–875. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Zimmerman, J.N.; Custodio, W.; Xiao, Y.; Basiri, T.; Hatibovic-Kofman, S.; Siqueira, W.L. Proteomic evaluation of acquired enamel pellicle during in vivo formation. PLoS ONE 2013, 8, e67919. [Google Scholar] [CrossRef] [Green Version]
- Vitorino, R.; Calheiros-Lobo, M.J.; Duarte, J.A.; Domingues, P.M.; Amado, F.M. Peptide profile of human acquired enamel pellicle using MALDI tandem MS. J. Sep. Sci. 2008, 31, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Lamkin, M.S.; Oppenheim, F.G. Pellicle precursor protein crosslinking characterization of an adduct between acidic proline-rich protein (PRP-1) and statherin generated by transglutaminase. J. Dent. Res. 2000, 79, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Won, S.H.; Kho, H.S.; Kim, Y.K.; Chung, S.C.; Lee, S.W. Analysis of residual saliva and minor salivary gland secretions. Arch. Oral Biol. 2001, 46, 619–624. [Google Scholar] [CrossRef]
- Smullen, J.; Koutsou, G.A.; Foster, H.A.; Zumbe, A.; Storey, D.M. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007, 41, 342–349. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinada, K.; Tagashira, M.; Watanabe, H.; Sopapornamorn, P.; Kanayama, A.; Kanda, T.; Ikeda, M.; Kawaguchi, Y. Hop bract polyphenols reduced three-day dental plaque regrowth. J. Dent. Res. 2007, 86, 848–851. [Google Scholar] [CrossRef] [Green Version]
- Philip, N.; Leishman, S.; Walsh, L. Potential Role for Natural Products in Dental Caries Control. Oral Health Prev. Dent. 2019, 17, 479–485. [Google Scholar] [CrossRef]
- Neto, C.C.; Penndorf, K.A.; Feldman, M.; Meron-Sudai, S.; Zakay-Rones, Z.; Steinberg, D.; Fridman, M.; Kashman, Y.; Ginsburg, I.; Ofek, I.; et al. Characterization of non-dialyzable constituents from cranberry juice that inhibit adhesion, co-aggregation and biofilm formation by oral bacteria. Food Funct. 2017, 8, 1955–1965. [Google Scholar] [CrossRef]
- Karygianni, L.; Al-Ahmad, A.; Argyropoulou, A.; Hellwig, E.; Anderson, A.C.; Skaltsounis, A.L. Natural Antimicrobials and Oral Microorganisms: A Systematic Review on Herbal Interventions for the Eradication of Multispecies Oral Biofilms. Front. Microbiol. 2016, 6, 1529. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-level antimicrobial efficacy of representative Mediterranean natural plant extracts against oral microorganisms. Biomed Res. Int. 2014, 2014, 839019. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, T.; Zhan, L.; Zhou, X. The effects of tea polyphenols on the adherence of cariogenic bacterium to the collagen in vitro. Hua Xi Kou Qiang Yi Xue Za Zhi 2000, 18, 340–342. [Google Scholar] [PubMed]
- Xiao, Y.; Liu, T.; Zhan, L.; Zhou, X. The effects of tea polyphenols on the adherence of cariogenic bacterium to the salivary acquired pellicle in vitro. Hua Xi Kou Qiang Yi Xue Za Zhi 2000, 18, 336–339. [Google Scholar] [PubMed]
- Duarte, S.; Gregoire, S.; Singh, A.P.; Vorsa, N.; Schaich, K.; Bowen, W.H.; Koo, H. Inhibitory effects of cranberry polyphenols on formation and acidogenicity of Streptococcus mutans biofilms. FEMS Microbiol. Lett. 2006, 257, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Thimothe, J.; Bonsi, I.A.; Padilla-Zakour, O.I.; Koo, H. Chemical characterization of red wine grape (Vitis vinifera and Vitis interspecific hybrids) and pomace phenolic extracts and their biological activity against Streptococcus mutans. J. Agric. Food Chem. 2007, 55, 10200–10207. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hamada, S.; Ooshima, T. Molecular analysis of the inhibitory effects of oolong tea polyphenols on glucan-binding domain of recombinant glucosyltransferases from Streptococcus mutans MT8148. FEMS Microbiol. Lett. 2003, 228, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Nakahara, K.; Kawabata, S.; Ono, H.; Ogura, K.; Tanaka, T.; Ooshima, T.; Hamada, S. Inhibitory Effect of Oolong Tea Polyphenols on Glucosyltransferases of Mutans Streptococci. Appl. Environ. Microb. 1993, 59, 968–973. [Google Scholar] [CrossRef] [Green Version]
- Ooshima, T.; Minami, T.; Aono, W.; Izumitani, A.; Sobue, S.; Fujiwara, T.; Kawabata, S.; Hamada, S. Oolong Tea Polyphenols Inhibit Experimental Dental-Caries in Spf Rats Infected with Mutans Streptococci. Caries Res. 1993, 27, 124–129. [Google Scholar] [CrossRef]
- Percival, R.S.; Devine, D.A.; Duggal, M.S.; Chartron, S.; Marsh, P.D. The effect of cocoa polyphenols on the growth, metabolism, and biofilm formation by Streptococcus mutans and Streptococcus sanguinis. Eur. J. Oral Sci. 2006, 114, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Schiller, N.L.; Kahng, H.Y.; Oh, K.H. Cellular responses and proteomic analysis of Escherichia coli exposed to green tea polyphenols. Curr. Microbiol. 2007, 55, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Kensche, A.; Buschbeck, E.; Konig, B.; Koch, M.; Kirsch, J.; Hannig, C.; Hannig, M. Effect of fluoride mouthrinses and stannous ions on the erosion protective properties of the in situ pellicle. Sci. Rep. 2019, 9, 5336. [Google Scholar] [CrossRef]
- Bernabe, E.; Vehkalahti, M.M.; Sheiham, A.; Lundqvist, A.; Suominen, A.L. The Shape of the Dose-Response Relationship between Sugars and Caries in Adults. J. Dent. Res. 2016, 95, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Van der Mei, H.C.; Engels, E.; de Vries, J.; Busscher, H.J. Effects of amine fluoride on biofilm growth and salivary pellicles. Caries Res. 2008, 42, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Crall, J.J. Improving oral health and oral health care delivery for children. J. Calif. Dent. Assoc. 2011, 39, 90–100. [Google Scholar] [PubMed]
- Martinez-Hernandez, M.; Reda, B.; Hannig, M. Chlorhexidine rinsing inhibits biofilm formation and causes biofilm disruption on dental enamel in situ. Clin. Oral Investig. 2020, 24, 3843–3853. [Google Scholar] [CrossRef] [PubMed]
- Moshrefi, A. Chlorhexidine. J. West. Soc. Periodontol. Periodontal Abstr. 2002, 50, 5–9. [Google Scholar]
- Keijser, B.J.; Zaura, E.; Huse, S.M.; van der Vossen, J.M.; Schuren, F.H.; Montijn, R.C.; ten Cate, J.M.; Crielaard, W. Pyrosequencing analysis of the oral microflora of healthy adults. J Dent Res. 2008, 87, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.P.; Hoth-Hannig, W.; Deng, S.L.; Jin, X.T.; Fu, B.P.; Hannig, M. The effect of polyphenol-containing solutions on in situ biofilm formation on enamel and dentin. J. Dent. 2020, 102, 103482. [Google Scholar] [CrossRef] [PubMed]
- Schestakow, A.; Hannig, M. Effects of Experimental Agents Containing Tannic Acid or Chitosan on the Bacterial Biofilm Formation in Situ. Biomolecules 2020, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Ebert, N.; Kensche, A.; Lock, S.; Hadiwikarta, W.W.; Hansch, A.; Dorr, W.; Krause, M.; Hannig, C.; Baumann, M. Results of a randomized controlled phase III trial: Efficacy of polyphenol-containing cystus (R) tea mouthwash solution for the reduction of mucositis in head and neck cancer patients undergoing external beam radiotherapy. Strahlenther. Onkol. 2021, 197, 63–73. [Google Scholar] [CrossRef]


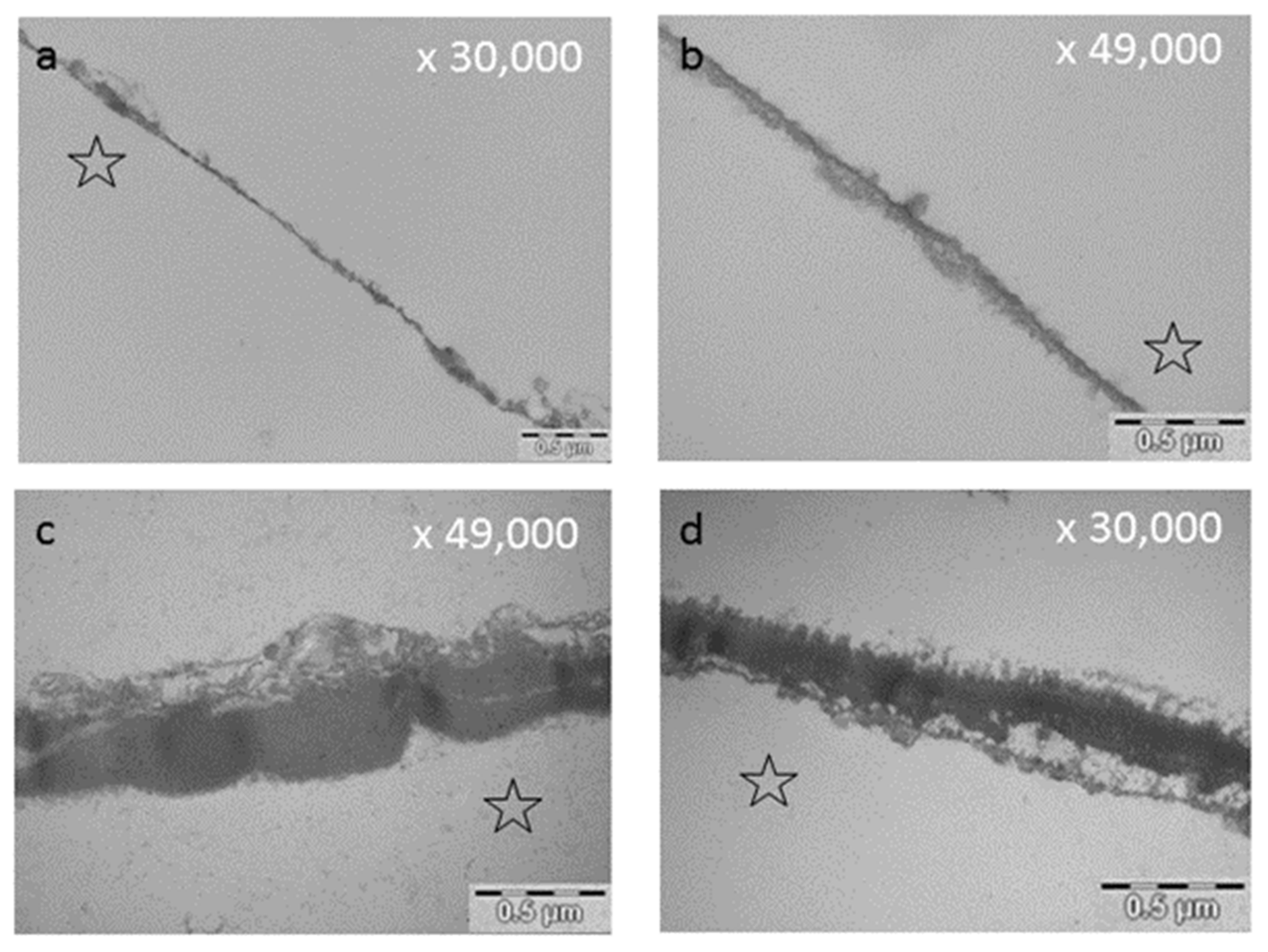
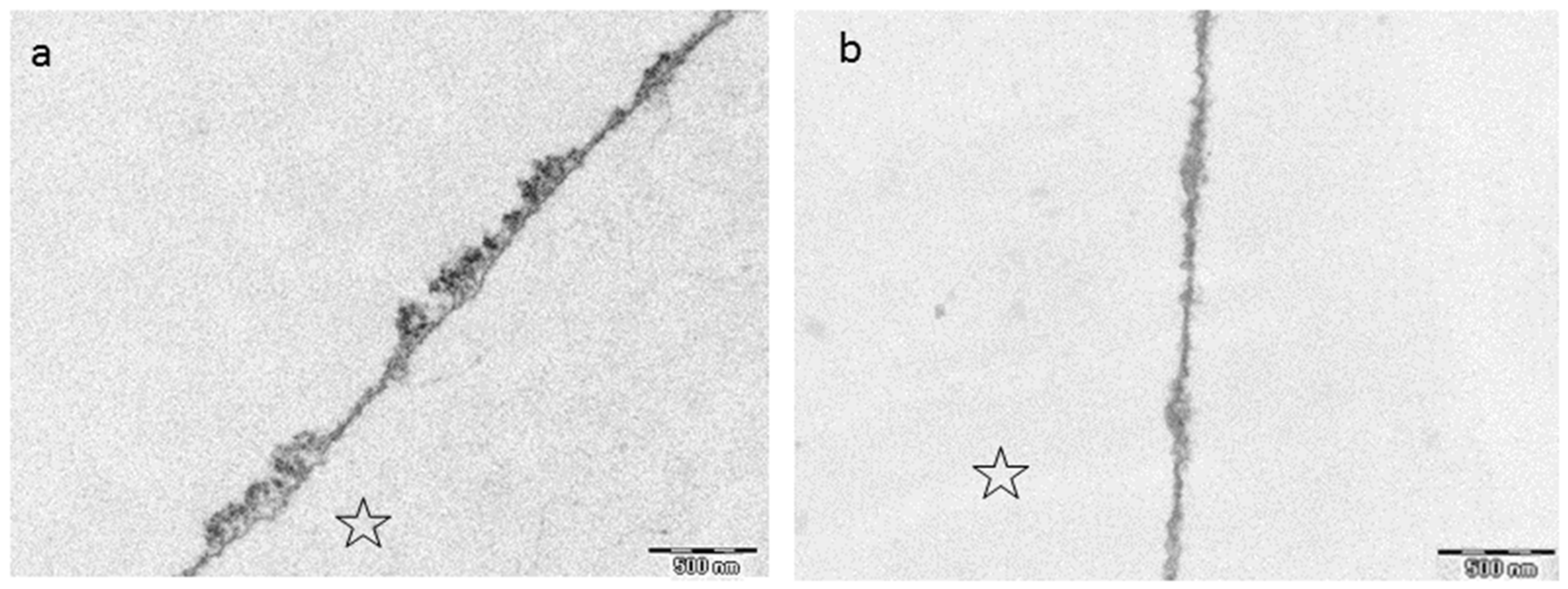

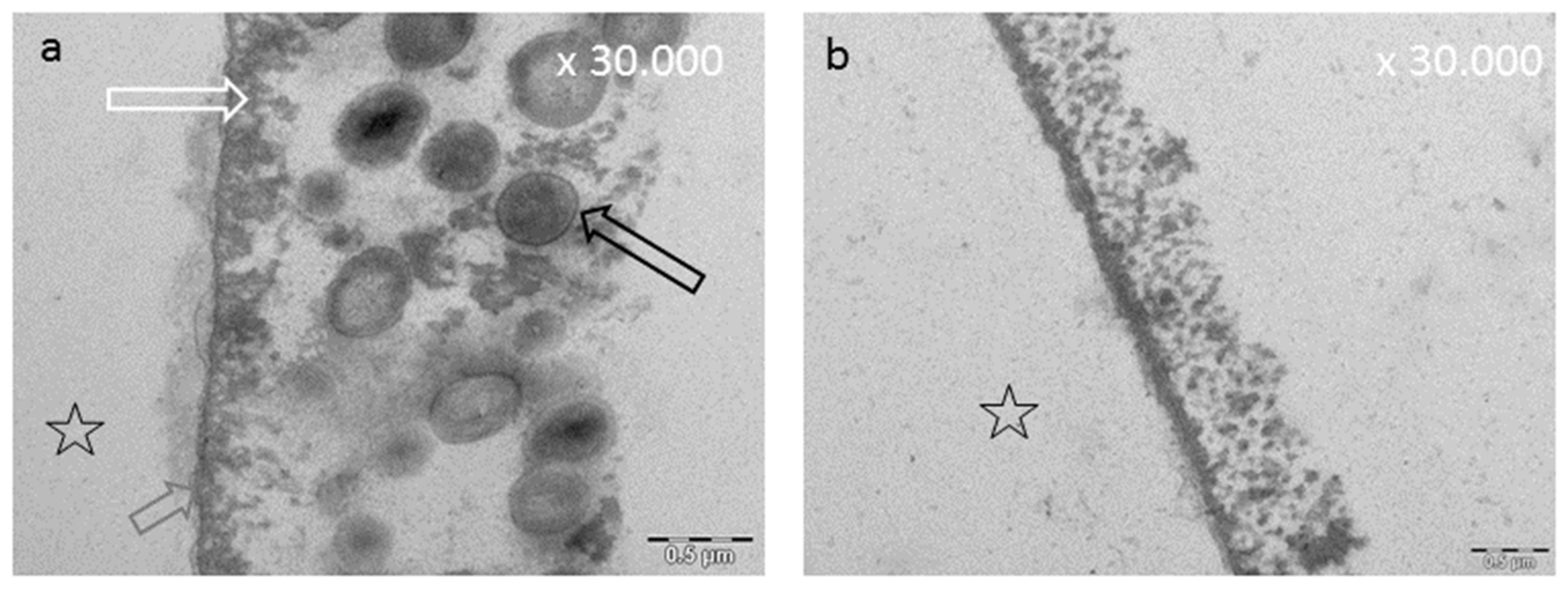
| Food or Beverage | References | |
|---|---|---|
| Vegetables | Different Tomato Varieties | [24,25,26] |
| Pepper | [25] | |
| Eggplant | [25] | |
| Olive Pomace | [27] | |
| Potato | [25,26] | |
| Lettuce, Onion | [26] | |
| Fruits | Fruit and Fruit Juices | [28,29] |
| Different Fruits | [30] | |
| Pomegranate | [31,32] | |
| Mango | [33] | |
| Strawberry | [34,35,36] | |
| Blueberry | [34,35,36] | |
| Raspberry | [35] | |
| Blackberry | [35] | |
| Cranberry | [35] | |
| Cherry | [26] | |
| Apple | [26] | |
| Nuts | Raw Nuts | [37,38] |
| Legumes | Beans | [39] |
| Chickpea, Green Gram, Pearl Millet, Finger Millet | [40] | |
| Tea | Black Tea | [41,42,43,44] |
| Green Tea | [41,42,43,44,45] | |
| White Tea | [45] | |
| Coffee | [41,44] | |
| Wine | Red and White Wine | [46] |
| Tea Variety | Polyphenol | Effect | Reference | Study Design |
|---|---|---|---|---|
| Green Tea | EGCG | - Increase in pellicle thickness and density | [68] | In situ |
| - Increase in pellicle thickness and density - No chance of charge of the tooth surface | [181] | In situ | ||
| Whole Green Tea | - Significant reduction in initial bacterial adhesion after 30 and 120 min of oral exposure | [182] | In situ | |
| Black Tea | Whole BlackTea, ECG, EGCG, Theaflavin | - Absorption of ECG, EGCG, theaflavin on the tooth surface, modification of the in-vitro pellicle | [179] | In vitro |
| - Modification of pellicle structure due to cross-linking between polyphenolic compounds and the pellicle as well as salivary proteins | [180] | In vitro | ||
| Catechins, Theaflavins | - Binding to salivary | [199] | In vitro, in vivo | |
| Whole Black Tea | - Significant reduction in the initial bacterial adhesion after 30 and 120 min | [182] | In situ | |
| - Significant reduction in immobilized lysozyme activity after 3 and 30 min | [200] | In situ |
| Tea Variety | Polyphenol | Effect | Reference | Study Design |
|---|---|---|---|---|
| Cistus Incanus | Ellagitannins, Flavonoid Group | - Antibacterial effects In-vitro-Live/Dead assay - In situ: Reduced initial bacterial colonisation | [63] | In vitro, in situ |
| Hydrolysable Tannins | - Antibacterial effect against S. mutans | [201] | In vitro | |
| Quercetin, Myrecetin, Gallic Acid | [202] | In vitro | ||
| Catechins | - Prevention of bacterial adhesion - Inhibition of GTF - Inhibition of amylase | [203], [204] | In vitro | |
| Whole Extract | - Significant reduction in the initial bacterial adhesion after 30 and 120 min | [182] | In situ | |
| - No impact of lysozyme and the immobilized enzymes | [62], [198] | In situ | ||
| Inula Viscosa | Flavonoids | - Antibacterial activity against cariogenic bacteria | [211] | In vitro |
| Luteolin | - Antimicrobial effect | [206] | In vitro | |
| Apigenin | - Antibacterial activity | [208] | In vitro | |
| [209] | In vivo | |||
| Whole Extract | - Antiadherent effect - Significant reduction in adherent bacteria | [63], [77], [182] | In situ | |
| Fragaria Vesca, Hamamelis, Tormentil | - Prolonged antiadherent effect on bacterial colonization, thickening and an enhanced density of ultrastructure | [149] | In situ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flemming, J.; Meyer-Probst, C.T.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Preventive Applications of Polyphenols in Dentistry—A Review. Int. J. Mol. Sci. 2021, 22, 4892. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094892
Flemming J, Meyer-Probst CT, Speer K, Kölling-Speer I, Hannig C, Hannig M. Preventive Applications of Polyphenols in Dentistry—A Review. International Journal of Molecular Sciences. 2021; 22(9):4892. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094892
Chicago/Turabian StyleFlemming, Jasmin, Clara Theres Meyer-Probst, Karl Speer, Isabelle Kölling-Speer, Christian Hannig, and Matthias Hannig. 2021. "Preventive Applications of Polyphenols in Dentistry—A Review" International Journal of Molecular Sciences 22, no. 9: 4892. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094892






