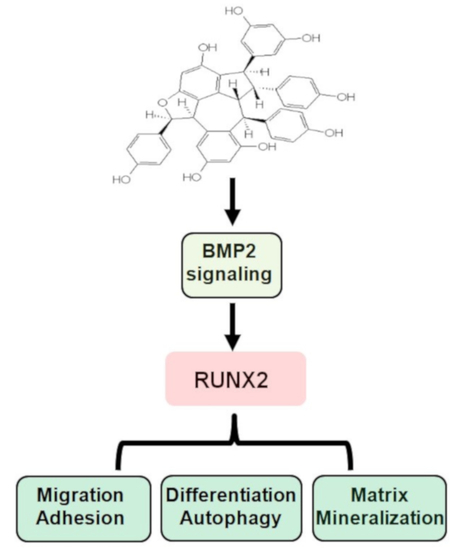
Suffruticosol B Is an Osteogenic Inducer through Osteoblast Differentiation, Autophagy, Adhesion, and Migration
Abstract
:1. Introduction
2. Results
2.1. Isolation of Suf-B from P. suffruticosa Fruits
2.2. Suf-B Facilitates Osteoblast Differentiation and Mineralization
2.3. Suf-B Facilitates BMP2-Smad1/5/8 Signaling and MAPKs Molecules
2.4. Suf-B Facilitates RUNX2 Expression and Autophagy in Osteoblast Differentiation
2.5. Suf-B Facilitates Osteoblast-Mediated Bone-Forming Phenotypes
3. Discussion
4. Materials and Methods
4.1. Material, Procedures, and Isolation
4.2. Pre-Osteoblast and Differentiation
4.3. MTT Assay
4.4. ALP and ARS Assays
4.5. Western Blot Analysis
4.6. Immunocytochemistry
4.7. Autophagy Detection
4.8. Adhesion and Migration Assays
4.9. Phalloidin and DRAQ5 Staining
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| ARS | Alizarin red S staining |
| L-AA | L-ascorbic acid |
| MAPKs | Osteogenic supplement medium |
| P. suffruticosa | Paeonia suffruticosa |
| RUNX2 | Runt-related transcription factor 2 |
| Suf-B | Suffruticosol B |
References
- Suo, Z.L.; Li, W.Y.; Yao, J.; Zhang, H.J.; Zhang, Z.M.; Zhao, D.X. Applicability of leaf morphology and intersimple sequence repeat markers in classification of tree peony (Paeoniaceae) cultivars. Hortscience 2005, 40, 329–334. [Google Scholar] [CrossRef] [Green Version]
- He, C.N.; Peng, Y.; Xu, L.J.; Liu, Z.A.; Gu, J.; Zhong, A.G.; Xiao, P.G. Three New Oligostilbenes from the Seeds of Paeonia suffruticosa. Chem. Pharm. Bull. 2010, 58, 843–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, M.; Ohta, T.; Kawaguchi, A.; Matsuda, H. Bioactive constituents of Chinese natural medicines. V. Radical scavenging effect of Moutan Cortex. (1): Absolute stereostructures of two monoterpenes, paeonisuffrone and paeonisuffral. Chem. Pharm. Bull. 2000, 48, 1327–1331. [Google Scholar] [CrossRef] [Green Version]
- Ryu, G.; Park, E.K.; Joo, J.H.; Lee, B.H.; Choi, B.W.; Jung, D.S.; Lee, N.H. A new antioxidant monoterpene glycoside, alpha-benzoyloxypaeoniflorin from Paeonia suffruticosa. Arch. Pharm. Res. 2001, 24, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Seo, C.S.; Lee, K.S.; Kim, H.J.; Chang, H.W.; Jung, J.S.; Song, D.K.; Son, J.K. Protective constituents against sepsis in mice from the root cortex of Paeonia suffruticosa. Arch. Pharm. Res. 2004, 27, 1123–1126. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, C.; Sun, Q.L.; Li, F.W.; Liu, J.H.; Zheng, C.C. Isolation and purification of four flavonoid constituents from the flowers of Paeonia suffruticosa by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1075, 127–131. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Raorane, C.J.; Ryu, S.Y.; Shim, J.J.; Lee, J. The anti-biofilm and anti-virulence activities of trans-resveratrol and oxyresveratrol against uropathogenic Escherichia coli. Biofouling 2019, 35, 758–767. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Chen, B.; Tong, L.; Zhang, Y. Tetrahydroxy stilbene glucoside protected against diabetes-induced osteoporosis in mice with streptozotocin-induced hyperglycemia. Phytother. Res. 2019, 33, 442–451. [Google Scholar] [CrossRef]
- Cai, T.; Cai, Y. cis-Ampelopsin E, a stilbene isolated from the seeds of Paeonia suffruticosa, inhibits lipopolysaccharide-stimulated nitric oxide production in RAW 264.7 macrophages via blockade of nuclear factor-kappa B signaling pathway. Biol. Pharm. Bull. 2011, 34, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
- Simoni, D.; Invidiata, F.P.; Eleopra, M.; Marchetti, P.; Rondanin, R.; Baruchello, R.; Grisolia, G.; Tripathi, A.; Kellogg, G.E.; Durrant, D.; et al. Design, synthesis and biological evaluation of novel stilbene-based antitumor agents. Bioorg. Med. Chem. 2009, 17, 512–522. [Google Scholar] [CrossRef]
- Almosnid, N.M.; Gao, Y.; He, C.; Park, H.S.; Altman, E. In vitro antitumor effects of two novel oligostilbenes, cis- and trans-suffruticosol D, isolated from Paeonia suffruticosa seeds. Int. J. Oncol. 2016, 48, 646–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, S.D.; Whiting, P.; Dinan, L.; Sik, V.; Rees, H.H. Identification and ecdysteroid antagonist activity of three resveratrol trimers (suffruticosols A, B and C) from Paeonia suffruticosa. Tetrahedron 1999, 55, 513–524. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, E.J.; Bae, S.J.; Shim, S.M.; Park, H.D.; Rhee, C.H.; Park, J.H.; Choi, S.W. Cytotoxic and antimutagenic stilbenes from seeds of Paeonia lactiflora. Arch. Pharm. Res. 2002, 25, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Park, Y.H.; Choi, S.W.; Yang, E.K.; Lee, W.J. Resveratrol derivatives potently induce apoptosis in human promyelocytic leukemia cells. Exp. Mol. Med. 2003, 35, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Deng, R.X.; Liu, P.; Hu, J.X.; Niu, W.T.; Gao, J.Y. Secondary Metabolite Mapping Identifies Peony Episperm Inhibitors of Human Hepatoma Cells. Nat. Prod. Commun. 2019, 14, 1934578X19860313. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, E.J.; Cho, S.H.; Chung, S.K.; Park, H.D.; Choi, S.W. Antioxidative activity of resveratrol and its derivatives isolated from seeds of Paeonia lactiflora. Biosci. Biotech. Bioch. 2002, 66, 1990–1993. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.G.; Li, M.Z.; Qian, D.W.; Liu, Z.A.; Shu, Q.Y. Phytochemical profiles and the hypoglycemic effects of tree peony seed coats. Food Funct. 2021, 12, 11777–11789. [Google Scholar] [CrossRef]
- Karsenty, G. The complexities of skeletal biology. Nature 2003, 423, 316–318. [Google Scholar] [CrossRef]
- Gu, S.M.; Park, M.H.; Yun, H.M.; Han, S.B.; Oh, K.W.; Son, D.J.; Yun, J.S.; Hong, J.T. CCR5 knockout suppresses experimental autoimmune encephalomyelitis in C57BL/6 mice. Oncotarget 2016, 7, 15382–15393. [Google Scholar] [CrossRef] [Green Version]
- Guntur, A.R.; Rosen, C.J. The skeleton: A multi-functional complex organ: New insights into osteoblasts and their role in bone formation: The central role of PI3Kinase. J. Endocrinol. 2011, 211, 123–130. [Google Scholar] [CrossRef]
- Seeman, E. Pathogenesis of bone fragility in women and men. Lancet 2002, 359, 1841–1850. [Google Scholar] [CrossRef]
- Marie, P.J. Osteoblast dysfunctions in bone diseases: From cellular and molecular mechanisms to therapeutic strategies. Cell. Mol. Life Sci. 2015, 72, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef] [Green Version]
- Javed, A.; Chen, H.; Ghori, F.Y. Genetic and transcriptional control of bone formation. Oral Maxillofac. Surg. Clin. N. Am. 2010, 22, 283–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsenty, G.; Kronenberg, H.M.; Settembre, C. Genetic control of bone formation. Annu. Rev. Cell Dev. Biol. 2009, 25, 629–648. [Google Scholar] [CrossRef]
- Zheng, X.; Dai, J.; Zhang, H.; Ge, Z. MicroRNA-221 promotes cell proliferation, migration, and differentiation by regulation of ZFPM2 in osteoblasts. Braz. J. Med. Biol. Res. 2018, 51, e7574. [Google Scholar] [CrossRef]
- Histing, T.; Stenger, D.; Kuntz, S.; Scheuer, C.; Tami, A.; Garcia, P.; Holstein, J.H.; Klein, M.; Pohlemann, T.; Menger, M.D. Increased osteoblast and osteoclast activity in female senescence-accelerated, osteoporotic SAMP6 mice during fracture healing. J. Surg. Res. 2012, 175, 271–277. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.L.; Yuan, Y.; Tu, J.; Zou, G.M.; Li, Q. Opposing TNF-alpha/IL-1beta- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation. Cell Death Dis. 2014, 5, e1187. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.M.; Park, K.R.; Quang, T.H.; Oh, H.; Hong, J.T.; Kim, Y.C.; Kim, E.C. 2,4,5-Trimethoxyldalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/beta-catenin pathway. Cell Death Dis. 2015, 6, e1819. [Google Scholar] [CrossRef]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golub, E.E.; Harrison, G.; Taylor, A.G.; Camper, S.; Shapiro, I.M. The role of alkaline phosphatase in cartilage mineralization. Bone Miner. 1992, 17, 273–278. [Google Scholar] [CrossRef]
- Yun, H.M.; Park, K.R.; Hong, J.T.; Kim, E.C. Peripheral serotonin-mediated system suppresses bone development and regeneration via serotonin 6 G-protein-coupled receptor. Sci. Rep. 2016, 6, 30985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Ogata, Y. Bone sialoprotein and its transcriptional regulatory mechanism. J. Periodontal Res. 2008, 43, 127–135. [Google Scholar] [CrossRef]
- Wennberg, C.; Hessle, L.; Lundberg, P.; Mauro, S.; Narisawa, S.; Lerner, U.H.; Millan, J.L. Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J. Bone Miner. Res. 2000, 15, 1879–1888. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, N.X.; Luo, Y.; Yu, C.Y.; Xiao, J.H. Ganoderal A effectively induces osteogenic differentiation of human amniotic mesenchymal stem cells via cross-talk between Wnt/beta-catenin and BMP/SMAD signaling pathways. Biomed. Pharmacother. 2020, 123, 109807. [Google Scholar] [CrossRef]
- Shen, Y.S.; Chen, X.J.; Wuri, S.N.; Yang, F.; Pang, F.X.; Xu, L.L.; He, W.; Wei, Q.S. Polydatin improves osteogenic differentiation of human bone mesenchymal stem cells by stimulating TAZ expression via BMP2-Wnt/beta-catenin signaling pathway. Stem Cell Res. Ther. 2020, 11, 204. [Google Scholar] [CrossRef]
- Chung, H.J.; Kim, W.K.; Oh, J.; Kim, M.R.; Shin, J.S.; Lee, J.; Ha, I.H.; Lee, S.K. Anti-Osteoporotic Activity of Harpagoside by Upregulation of the BMP2 and Wnt Signaling Pathways in Osteoblasts and Suppression of Differentiation in Osteoclasts. J. Nat. Prod. 2017, 80, 434–442. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, B.; Huang, L.; Wu, H.; Huang, J.; Liang, T.; Liu, H.; Zheng, L.; Zhao, J. Andrographolide Exerts Pro-Osteogenic Effect by Activation of Wnt/beta-Catenin Signaling Pathway in Vitro. Cell Physiol. Biochem. 2015, 36, 2327–2339. [Google Scholar] [CrossRef]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef]
- Park, K.R.; Park, J.E.; Kim, B.; Kwon, I.K.; Hong, J.T.; Yun, H.M. Calycosin-7-O-beta-Glucoside Isolated from Astragalus membranaceus Promotes Osteogenesis and Mineralization in Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 11362. [Google Scholar] [CrossRef]
- Park, K.R.; Kim, B.; Lee, J.Y.; Moon, H.J.; Kwon, I.K.; Yun, H.M. Effects of Scoparone on differentiation, adhesion, migration, autophagy and mineralization through the osteogenic signalling pathways. J. Cell Mol. Med. 2022, 26, 4520–4529. [Google Scholar] [CrossRef] [PubMed]
- Styrkarsdottir, U.; Cazier, J.B.; Kong, A.; Rolfsson, O.; Larsen, H.; Bjarnadottir, E.; Johannsdottir, V.D.; Sigurdardottir, M.S.; Bagger, Y.; Christiansen, C.; et al. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 2003, 1, E69. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kim, Y.J.; Kim, H.J.; Park, H.D.; Kang, A.R.; Kyung, H.M.; Sung, J.H.; Wozney, J.M.; Ryoo, H.M. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-beta 1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J. Biol. Chem. 2003, 278, 34387–34394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.R.; Park, J.I.; Lee, S.; Yoo, K.; Kweon, G.R.; Kwon, I.K.; Yun, H.M.; Hong, J.T. Chi3L1 is a therapeutic target in bone metabolism and a potential clinical marker in patients with osteoporosis. Pharmacol. Res. 2022, 184, 106423. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.B.; Song, Y.; Hwang, J.K. Kirenol stimulates osteoblast differentiation through activation of the BMP and Wnt/beta-catenin signaling pathways in MC3T3-E1 cells. Fitoterapia 2014, 98, 59–65. [Google Scholar] [CrossRef]
- Park, K.R.; Lee, J.Y.; Kim, B.M.; Kang, S.W.; Yun, H.M. TMARg, a Novel Anthraquinone Isolated from Rubia cordifolia Nakai, Increases Osteogenesis and Mineralization through BMP2 and beta-Catenin Signaling. Int. J. Mol. Sci. 2020, 21, 5332. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Lee, J.Y.; Cho, M.; Yun, H.M. Ziyuglycoside I Upregulates RUNX2 through ERK1/2 in Promoting Osteoblast Differentiation and Bone Mineralization. Am. J. Chin. Med. 2021, 49, 883–900. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- di Giacomo, V.; Cataldi, A.; Sancilio, S. Biological Factors, Metals, and Biomaterials Regulating Osteogenesis through Autophagy. Int. J. Mol. Sci. 2020, 21, 2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, I.M.; Layfield, R.; Lotz, M.; Settembre, C.; Whitehouse, C. Boning up on autophagy: The role of autophagy in skeletal biology. Autophagy 2014, 10, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Li, D.; Ma, Z.; Qian, Z.; Kang, X.; Jin, X.; Li, F.; Wang, X.; Chen, Q.; Sun, H.; et al. Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy 2018, 14, 1726–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.R.; Kim, S.E.; Baek, H.S.; Kim, B.J.; Kim, C.H.; Chung, I.K.; Park, B.S.; Shin, S.H. The role of kaempferol-induced autophagy on differentiation and mineralization of osteoblastic MC3T3-E1 cells. BMC Complement. Altern. Med. 2016, 16, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinisalo, M.; Karlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef] [Green Version]
- Infante, A.; Rodriguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Granero-Molto, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [Green Version]
- Delaisse, J.M. The reversal phase of the bone-remodeling cycle: Cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014, 3, 561. [Google Scholar] [CrossRef] [Green Version]
- Kalbacova, M.; Broz, A.; Kong, J.; Kalbac, M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon 2010, 48, 4323–4329. [Google Scholar] [CrossRef]
- Aryaei, A.; Jayatissa, A.H.; Jayasuriya, A.C. The effect of graphene substrate on osteoblast cell adhesion and proliferation. J. Biomed. Mater. Res. Part A 2014, 102, 3282–3290. [Google Scholar] [CrossRef]
- Tong, Z.; Liu, Y.; Xia, R.; Chang, Y.; Hu, Y.; Liu, P.; Zhai, Z.; Zhang, J.; Li, H. F-actin Regulates Osteoblastic Differentiation of Mesenchymal Stem Cells on TiO2 Nanotubes Through MKL1 and YAP/TAZ. Nanoscale Res. Lett. 2020, 15, 183. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Hong, X.; Li, Z.; Deng, C.X.; Fu, J. Acoustic tweezing cytometry enhances osteogenesis of human mesenchymal stem cells through cytoskeletal contractility and YAP activation. Biomaterials 2017, 134, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, H.M.; Kim, B.; Jeong, Y.H.; Hong, J.T.; Park, K.R. Suffruticosol A elevates osteoblast differentiation targeting BMP2-Smad/1/5/8-RUNX2 in pre-osteoblasts. Biofactors, 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Kim, E.C.; Hong, J.T.; Yun, H.M. Dysregulation of 5-hydroxytryptamine 6 receptor accelerates maturation of bone-resorbing osteoclasts and induces bone loss. Theranostics 2018, 8, 3087–3098. [Google Scholar] [CrossRef]
- Yun, H.M.; Kim, S.; Kim, H.J.; Kostenis, E.; Kim, J.I.; Seong, J.Y.; Baik, J.H.; Rhim, H. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J. Biol. Chem. 2007, 282, 5496–5505. [Google Scholar] [CrossRef] [Green Version]
- Park, K.R.; Leem, H.H.; Kwon, Y.J.; Kwon, I.K.; Hong, J.T.; Yun, H.M. Falcarindiol Stimulates Apoptotic and Autophagic Cell Death to Attenuate Cell Proliferation, Cell Division, and Metastasis through the PI3K/AKT/mTOR/p70S6K Pathway in Human Oral Squamous Cell Carcinomas. Am. J. Chin. Med. 2022, 50, 295–311. [Google Scholar] [CrossRef]
- Park, K.R.; Leem, H.H.; Cho, M.; Kang, S.W.; Yun, H.M. Effects of the amide alkaloid piperyline on apoptosis, autophagy, and differentiation of pre-osteoblasts. Phytomedicine 2020, 79, 153347. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-M.; Lee, J.Y.; Kim, B.; Park, K.-R. Suffruticosol B Is an Osteogenic Inducer through Osteoblast Differentiation, Autophagy, Adhesion, and Migration. Int. J. Mol. Sci. 2022, 23, 13559. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232113559
Yun H-M, Lee JY, Kim B, Park K-R. Suffruticosol B Is an Osteogenic Inducer through Osteoblast Differentiation, Autophagy, Adhesion, and Migration. International Journal of Molecular Sciences. 2022; 23(21):13559. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232113559
Chicago/Turabian StyleYun, Hyung-Mun, Joon Yeop Lee, Bomi Kim, and Kyung-Ran Park. 2022. "Suffruticosol B Is an Osteogenic Inducer through Osteoblast Differentiation, Autophagy, Adhesion, and Migration" International Journal of Molecular Sciences 23, no. 21: 13559. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms232113559