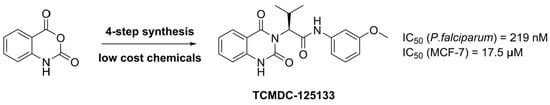
A Concise Synthesis towards Antimalarial Quinazolinedione TCMDC-125133 and Its Anti-Proliferative Activity against MCF-7
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. (–)-Ethyl (2-aminobenzoyl)-l-valinate (1)

3.2.2. (–)-Ethyl (S)-2-(2,4-dioxo-1,4-dihydroquinazolin-3(2H)-yl)-3-methylbutanoate (2)

3.2.3. (–)-(S)-2-(2,4-dioxo-1,4-dihydroquinazolin-3(2H)-yl)-3-methylbutanoic acid (3)

3.2.4. (–)-(S)-2-(2,4-dioxo-1,4-dihydroquinazolin-3(2H)-yl)-N-(3-methoxyphenyl)-3-methyl-butanamide (4) [TCMDC-125133]

3.3. Antimalarial Assay against P. falciparum 3D7
3.4. Antiproliferative Assay against MCF-7 and HCT-116
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birhan, Y.S.; Bekhit, A.A.; Hymete, A. In Vivo antimalarial evaluation of some 2,3-disubstituted-4(3H)-quinazolinone derivatives. BMC Res. Notes 2015, 8, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouhar, R.S.; Kamel, M.M. Synthesis and Reactions of Some New Quinazoline Derivatives forIn VitroEvaluation as Anticancer and Antimicrobial Agents. J. Heterocycl. Chem. 2018, 55, 2082–2089. [Google Scholar] [CrossRef]
- Tsuchihashi, H.; Nagatomo, T. Alpha-blocking potencies of antihypertensive agents (prazosin, terazosin, bunazosin, SGB-1534 and ketanserin) having with quinazoline or quinazolinedione as assessed by radioligand binding assay methods in rat brain. J. Pharmacobiodyn. 1989, 12, 170–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matharu, D.S.; Flaherty, D.P.; Simpson, D.S.; Schroeder, C.E.; Chung, D.; Yan, D.; Noah, J.W.; Jonsson, C.B.; White, E.L.; Aubé, J.; et al. Optimization of Potent and Selective Quinazolinediones: Inhibitors of Respiratory Syncytial Virus That Block RNA-Dependent RNA-Polymerase Complex Activity. J. Med. Chem. 2014, 57, 10314–10328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraka, M.M. Synthesis of novel 2,4 (1H, 3H)-quinazolinedione derivatives with analgesic and anti-inflammatory activities. Boll. Chim. Farm. 2001, 140, 90–96. [Google Scholar] [PubMed]
- Zhang, X.; Ding, Q.; Wang, J.; Yang, J.; Fan, X.; Zhang, G. Pd(ii)-Catalyzed [4 + 1 + 1] cycloaddition of simple o-aminobenzoic acids, CO and amines: Direct and versatile synthesis of diverse N-substituted quinazoline-2,4(1H,3H)-diones. Green Chem. 2021, 23, 526–535. [Google Scholar] [CrossRef]
- Banasr, M.; Hery, M.; Printemps, R.; Daszuta, A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 2004, 29, 450–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonier, B.; Arseneault, M.; Lavigne, C.; Ouellette, R.J.; Vaillancourt, C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem. Biophys. Res. Commun. 2006, 343, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, S.H.; Ahangari, G.; Deezagi, A. Alternative Viewpoint Against Breast Cancer Based on Selective Serotonin Receptors 5HTR3A and 5HTR2A Antagonists that can Mediate Apoptosis in MCF-7 Cell Line. Curr. Drug Discov. Technol. 2015, 12, 240–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, H. Accelerating Antimalarial Drug Discovery through Repositioning; University of Salford: Salford, UK, 2015. [Google Scholar]
- Calderon, F.; Barros, D.; Bueno, J.M.; Coteron, J.M.; Fernandez, E.; Gamo, F.J.; Lavandera, J.L.; Leon, M.L.; Macdonald, S.J.; Mallo, A.; et al. An Invitation to Open Innovation in Malaria Drug Discovery: 47 Quality Starting Points from the TCAMS. ACS Med. Chem. Lett. 2011, 2, 741–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almela, M.J.; Lozano, S.; Lelievre, J.; Colmenarejo, G.; Coteron, J.M.; Rodrigues, J.; Gonzalez, C.; Herreros, E. A New Set of Chemical Starting Points with Plasmodium falciparum Transmission-Blocking Potential for Antimalarial Drug Discovery. PLoS ONE 2015, 10, e0135139. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Tsuboi, H.; Kanoda, M.; Mukai, K.; Kagara, K. The Process Development of a Novel Aldose Reductase Inhibitor, FK366. Part 1. Improvement of Discovery Process and New Syntheses of 1-Substituted Quinazolinediones. Org. Process Res. Dev. 2003, 7, 700–706. [Google Scholar] [CrossRef]
- Ismail, M.A.H.; Barker, S.; Abou El Ella, D.A.; Abouzid, K.A.M.; Toubar, R.A.; Todd, M.H. Design and Synthesis of New Tetrazolyl- and Carboxy-biphenylylmethyl-quinazolin-4-one Derivatives as Angiotensin II AT1 Receptor Antagonists. J. Med. Chem. 2006, 49, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Nencini, A.; Pratelli, C.; Quinn, J.M.; Salerno, M.; Tunici, P.; De Robertis, A.; Valensin, S.; Mennillo, F.; Rossi, M.; Bakker, A.; et al. Structure-activity relationship and properties optimization of a series of quinazoline-2,4-diones as inhibitors of the canonical Wnt pathway. Eur. J. Med. Chem. 2015, 95, 526–545. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.-J.; Park, J.; Kim, B.; Lee, H.-G.; Kang, S.-B.; Sung, G.; Kim, J.-J.; Lee, S.-G. tert-Butoxide-Assisted Amidation of Esters under Green Conditions. Synthesis 2011, 44, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Carpino, L.A.; Imazumi, H.; Foxman, B.M.; Vela, M.J.; Henklein, P.; El-Faham, A.; Klose, J.; Bienert, M. Comparison of the Effects of 5- and 6-HOAt on Model Peptide Coupling Reactions Relative to the Cases for the 4- and 7-Isomers. Org. Lett. 2000, 2, 2253–2256. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoensutthivarakul, S.; Lohawittayanan, D.; Kanjanasirirat, P.; Jearawuttanakul, K.; Seemakhan, S.; Borwornpinyo, S.; Phanchana, M. A Concise Synthesis towards Antimalarial Quinazolinedione TCMDC-125133 and Its Anti-Proliferative Activity against MCF-7. Molbank 2022, 2022, M1358. https://0-doi-org.brum.beds.ac.uk/10.3390/M1358
Charoensutthivarakul S, Lohawittayanan D, Kanjanasirirat P, Jearawuttanakul K, Seemakhan S, Borwornpinyo S, Phanchana M. A Concise Synthesis towards Antimalarial Quinazolinedione TCMDC-125133 and Its Anti-Proliferative Activity against MCF-7. Molbank. 2022; 2022(2):M1358. https://0-doi-org.brum.beds.ac.uk/10.3390/M1358
Chicago/Turabian StyleCharoensutthivarakul, Sitthivut, Duangporn Lohawittayanan, Phongthon Kanjanasirirat, Kedchin Jearawuttanakul, Sawinee Seemakhan, Suparerk Borwornpinyo, and Matthew Phanchana. 2022. "A Concise Synthesis towards Antimalarial Quinazolinedione TCMDC-125133 and Its Anti-Proliferative Activity against MCF-7" Molbank 2022, no. 2: M1358. https://0-doi-org.brum.beds.ac.uk/10.3390/M1358