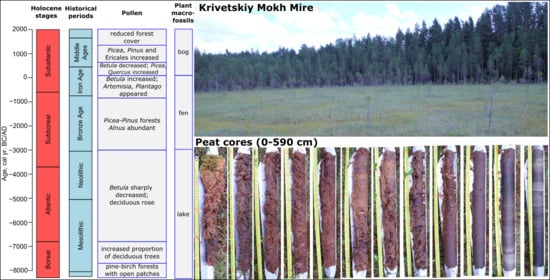
Peatland Development, Vegetation History, Climate Change and Human Activity in the Valdai Uplands (Central European Russia) during the Holocene: A Multi-Proxy Palaeoecological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region and Site
2.2. Sampling
2.3. Chronology
2.4. Loss on Ignition (LOI) and Peat Humification
2.5. Carbon (C), Nitrogen (N) and Stable Isotope Analyses
2.6. Macrocharcoal Analysis
2.7. Plant Macrofossils
2.8. Pollen Analysis
2.9. Testate Amoeba Analysis
2.10. Cladocera Analysis
2.11. Diatom Analysis
2.12. Data Visualization and Zonation
3. Results
3.1. Chronology and Biostratigraphy
3.2. Zone KM1 (700–500 cm; 8330–3000 BC): Lake Stage
3.3. Zone KM2 (500–410 cm, 3000–840 BC): Early Stages of Terrestrialization
3.4. Zone KM3 (410–330 cm, 840 BC–130 AD): Fen Stage
3.5. Zone KM4 (330–250 cm, 130–640 AD): Early Bog Stage
3.6. Zone KM5 (250–120 cm, 640–1350 AD): Wet Bog Stage
3.7. Zone KM6 (120–0 cm, 1350–2018 AD): Dry Bog Stage
4. Discussion
4.1. Regional Vegetation, Fire Regime and Climate Reconstruction
4.1.1. Boreal Stage (8300–6800 BC)
4.1.2. Atlantic Stage (6800–3700 BC)
4.1.3. Subboreal Stage (3700–600 BC)
4.1.4. Subatlantic Stage (600 BC–Present)
4.2. Peatland Development
4.2.1. Lake and Early Stages of Terrestrialization (8300–900 BC)
4.2.2. Fen and Early Stages of Ombrotrophication (900 BC–640 AD)
4.2.3. Bog (640 AD–until Present)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rydin, H.; Jeglum, J.K.; Bennett, K.D. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Minayeva, T.; Bragg, O.; Sirin, A. Peatland biodiversity and its restoration. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Cambridge University Press: Cambridge, UK, 2016; pp. 44–62. [Google Scholar]
- Yu, Z.; Loisel, J.; Brosseau, D.P.; Beilman, D.W.; Hunt, S.J. Global peatland dynamics since the Last Glacial Maximum. Geophys. Res. Lett. 2010, 37, L13402. [Google Scholar] [CrossRef]
- Kuhry, P.; Turunen, J. The postglacial development of boreal and subarctic peatlands. In Boreal Peatland Ecosystems; Springer: Berlin/Heidelberg, Germany, 2006; pp. 25–46. [Google Scholar]
- Klinger, L.F. The myth of the classic hydrosere model of bog succession. Arct. Alp. Res. 1996, 28, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Charman, D. Peatlands and Environmental Change; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Tsyganov, A.N.; Kupriyanov, D.A.; Babeshko, K.V.; Borisova, T.V.; Chernyshov, V.A.; Volkova, E.M.; Chekova, D.A.; Mazei, Y.A.; Novenko, E.Y. Autogenic and allogenic factors affecting development of a floating Sphagnum-dominated peat mat in a karst pond basin. Holocene 2019, 29, 120–129. [Google Scholar] [CrossRef]
- Gearey, B.; Fyfe, R. Peatlands as knowledge archives. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Bonn, A., Allott, T., Evans, M., Joosten, H., Stoneman, R., Eds.; Ecological Reviews; Cambridge University Press: Cambridge, UK, 2016; pp. 95–113. [Google Scholar] [CrossRef]
- Charman, D.J.; Beilman, D.W.; Blaauw, M.; Booth, R.K.; Brewer, S.; Chambers, F.M.; Christen, J.A.; Gallego-Sala, A.; Harrison, S.P.; Hughes, P.D.M.; et al. Climate-related changes in peatland carbon accumulation during the last millennium. Biogeosciences 2013, 10, 929–944. [Google Scholar] [CrossRef] [Green Version]
- McClymont, E.; Pendall, E.; Nichols, J. Stable isotopes and organic geochemistry in peat: Tools to investigate past hydrology, temperature and biogeochemistry. PAGES News 2010, 18, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Loisel, J.; Garneau, M.; Hélie, J.-F. Sphagnum δ13C values as indicators of palaeohydrological changes in a peat bog. Holocene 2010, 20, 285–291. [Google Scholar] [CrossRef]
- Blackford, J. Palaeoclimatic records from peat bogs. Trends Ecol. Evol. 2000, 15, 193–198. [Google Scholar] [CrossRef]
- Barber, K.E.; Chambers, F.M.; Maddy, D. Holocene palaeoclimates from peat stratigraphy: Macrofossil proxy climate records from three oceanic raised bogs in England and Ireland. Quat. Sci. Rev. 2003, 22, 521–539. [Google Scholar] [CrossRef]
- Mauquoy, D.; Hughes, P.; Geel, B.V. A protocol for plant macrofossil analysis of peat deposits. Mires Peat 2010, 7, 1–5. [Google Scholar]
- Payne, R.; Blackford, J. Peat humification and climate change: A multi-site comparison from mires in south-east Alaska. Mires Peat 2008, 3, 1–11. [Google Scholar]
- Amesbury, M.J.; Swindles, G.T.; Bobrov, A.; Charman, D.J.; Holden, J.; Lamentowicz, M.; Mallon, G.; Mazei, Y.; Mitchell, E.A.D.; Payne, R.J.; et al. Development of a new pan-European testate amoeba transfer function for reconstructing peatland palaeohydrology. Quat. Sci. Rev. 2016, 152, 132–151. [Google Scholar] [CrossRef] [Green Version]
- Swindles, G.T.; Morris, P.J.; Mullan, D.J.; Payne, R.J.; Roland, T.P.; Amesbury, M.J.; Lamentowicz, M.; Turner, T.E.; Gallego-Sala, A.; Sim, T.; et al. Widespread drying of European peatlands in recent centuries. Nat. Geosci. 2019, 12, 922–928. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Tsyganov, A.N.; Babeshko, K.V.; Payne, R.J.; Li, J.; Mazei, Y.A.; Olchev, A.V. Climatic moisture conditions in the north-west of the Mid-Russian Upland during the Holocene. Geogr. Environ. Sustain. 2019, 12, 188–202. [Google Scholar] [CrossRef]
- Kaufman, D.; McKay, N.; Routson, C.; Erb, M.; Dätwyler, C.; Sommer, P.S.; Heiri, O.; Davis, B. Holocene global mean surface temperature, a multi-method reconstruction approach. Sci. Data 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Fyfe, R.; Brück, J.; Johnston, R.; Lewis, H.; Roland, T.; Wickstead, H. Historical context and chronology of Bronze Age land enclosure on Dartmoor, UK. J. Archaeol. Sci. 2008, 35, 2250–2261. [Google Scholar] [CrossRef]
- Kasin, I.; Blanck, Y.; Storaunet, K.O.; Rolstad, J.; Ohlson, M. The charcoal record in peat and mineral soil across a boreal landscape and possible linkages to climate change and recent fire history. Holocene 2013, 23, 1052–1065. [Google Scholar] [CrossRef]
- Wanner, H.; Beer, J.; Bütikofer, J.; Crowley, T.J.; Cubasch, U.; Flückiger, J.; Goosse, H.; Grosjean, M.; Joos, F.; Kaplan, J.O.; et al. Mid- to Late Holocene climate change: An overview. Quat. Sci. Rev. 2008, 27, 1791–1828. [Google Scholar] [CrossRef]
- Mann, M.E.; Zhang, Z.; Rutherford, S.; Bradley, R.S.; Hughes, M.K.; Shindell, D.; Ammann, C.; Faluvegi, G.; Ni, F. Global signatures and dynamical origins of the Little Ice Age and Medieval Climate Anomaly. Science 2009, 326, 1256–1260. [Google Scholar] [CrossRef] [Green Version]
- Lappalainen, E. Global Peat Resources; International Peat Society Finland: Jyskae, Finland, 1996; Volume 4. [Google Scholar]
- Novenko, E.Y.; Tsyganov, A.N.; Volkova, E.M.E.M.; Babeshko, K.V.; Lavrentiev, N.V.N.V.; Payne, R.J.; Mazei, Y.A.Y.A. The Holocene paleoenvironmental history of central European Russia reconstructed from pollen, plant macrofossil, and testate amoeba analyses of the Klukva peatland, Tula region. Quat. Res. 2015, 83, 459–468. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Tsyganov, A.N.N.; Rudenko, O.V.; Volkova, E.V.; Zuyganova, I.S.; Babeshko, K.V.K.V.; Olchev, A.V.; Losbenev, N.I.; Payne, R.J.R.J.; Mazei, Y.A. Mid- and late-Holocene vegetation history, climate and human impact in the forest-steppe ecotone of European Russia: New data and a regional synthesis. Biodivers. Conserv. 2016, 25, 1–20. [Google Scholar] [CrossRef]
- Payne, R.J.; Malysheva, E.; Tsyganov, A.; Pampura, T.; Novenko, E.Y.; Volkova, E.; Babeshko, K.; Mazei, Y. A multi-proxy record of Holocene environmental change, peatland development and carbon accumulation from Staroselsky Moch peatland, Russia. Holocene 2016, 26, 314–326. [Google Scholar] [CrossRef]
- Sillasoo, U.; Mauquoy, D.; Blundell, A.; Charman, D.; Blaauw, M.; Daniell, J.R.; Toms, P.; Newberry, J.; Chambers, F.M.; Karofeld, E. Peat multi-proxy data from Männikjärve bog as indicators of late Holocene climate changes in Estonia. Boreas 2007, 36, 20–37. [Google Scholar] [CrossRef]
- Gałka, M.; Miotk-Szpiganowicz, G.; Marczewska, M.; Barabach, J.; van der Knaap, W.O.; Lamentowicz, M. Palaeoenvironmental changes in Central Europe (NE Poland) during the last 6200 years reconstructed from a high-resolution multi-proxy peat archive. Holocene 2015, 25, 421–434. [Google Scholar] [CrossRef]
- Lamentowicz, M.; Gałka, M.; Lamentowicz, Ł.; Obremska, M.; Kühl, N.; Lücke, A.; Jassey, V.E.J. Reconstructing climate change and ombrotrophic bog development during the last 4000 years in northern Poland using biotic proxies, stable isotopes and trait-based approach. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 418, 261–277. [Google Scholar] [CrossRef] [Green Version]
- Tuittila, E.-S.; Juutinen, S.; Frolking, S.; Väliranta, M.; Laine, A.M.; Miettinen, A.; Seväkivi, M.-L.; Quillet, A.; Merilä, P. Wetland chronosequence as a model of peatland development: Vegetation succession, peat and carbon accumulation. Holocene 2012, 23, 25–35. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Volkova, E.; Nosova, N.; Zuganova, I. Late Glacial and Holocene landscape dynamics in the southern taiga zone of East European Plain according to pollen and macrofossil records from the Central Forest State Reserve (Valdai Hills, Russia). Quat. Int. 2009, 207, 93–103. [Google Scholar] [CrossRef]
- Nosova, M.; Severova, E.; Volkova, O. A 6500-year pollen record from the Polistovo-Lovatskaya Mire System (northwest European Russia). Vegetation dynamics and signs of human impact. Grana 2017, 56, 410–423. [Google Scholar] [CrossRef]
- Nosova, M.B.; Novenko, E.Y.; Severova, E.E.; Volkova, O.A. Vegetation and climate changes within and around the Polistovo-Lovatskaya mire system (Pskov Oblast, north-western Russia) during the past 10,500 years. Veg. Hist. Archaeobotany 2019, 28, 123–140. [Google Scholar] [CrossRef]
- Łuców, D.; Lamentowicz, M.; Obremska, M.; Arkhipova, M.; Kittel, P.; Łokas, E.; Mazurkevich, A.; Mróz, T.; Tjallingii, R.; Słowiński, M. Disturbance and resilience of a Sphagnum peatland in western Russia (Western Dvina Lakeland) during the last 300 years: A multiproxy, high-resolution study. Holocene 2020, 30, 1552–1566. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; FAO: Rome, Italy, 2015. [Google Scholar]
- Sinitsyna, G.V.; Lavrushin, Y.A.; Spiridonova, E.A.; Guskova, Y.G.; Raspopov, O.M.; Iosifidi, A.G. About chronology of archaeological materials and about age of accomodating deposits of multy layer site Baranova Gora in Tver’ oblast’. In Tverskoy Arheologicheskiy Sbornik; TTOM: Tver’, Russia, 2009; Volume 7, pp. 52–70. [Google Scholar]
- Timofeyev, V.I. Raskopki neoliticheskikh stoyanok v Kalininskoy oblasti i v Priladozhye. In Arkheologicheskiye Otkrytiya 1973 Goda; Rybakov, B.A., Ed.; Nauka: Moscow, Russia, 1974; p. 81. [Google Scholar]
- Kashkin, A.V. Archaeological Map of Russia. Tver’ Region. Part 4: Bologovskiy, Penoskiy, Udomelskiy and Firovskiy Districts; IA RAS: Moscow, Russia, 2012; Volume 4. [Google Scholar]
- Reimer, P.J.; Austin, W.E.N.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Bronk Ramsey, C.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere Radiocarbon Age Calibration Curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Blaauw, M. clam: Classical Age-Depth Modelling of Cores from Deposits; R Package Version 2.3.5; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=clam (accessed on 24 November 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Blaauw, M.; Christen, J.A. Rbacon: Age-Depth Modelling Using Bayesian Statistics; R Package Version 2.3.5; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=rbacon (accessed on 28 October 2020).
- Dean, W.E. Determination of carbonate and organic matter in calcareous sediments and sedimentary rocks by loss on ignition; comparison with other methods. J. Sediment. Res. 1974, 44, 242–248. [Google Scholar]
- Chambers, F.M.; Beilman, D.W.; Yu, Z. Methods for determining peat humification and for quantifying peat bulk density, organic matter and carbon content for palaeostudies of climate and peatland. Mires Peat 2011, 7, 1–10. [Google Scholar]
- Mooney, S.; Tinner, W. The analysis of charcoal in peat and organic sediments. Mires Peat 2011, 7, 1–18. [Google Scholar]
- Higuera, P.E.; Peters, M.E.; Brubaker, L.B.; Gavin, D.G. Understanding the origin and analysis of sediment-charcoal records with a simulation model. Quat. Sci. Rev. 2007, 26, 1790–1809. [Google Scholar] [CrossRef]
- Smith, A.J.E. The Moss Flora of Britain and Ireland; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Mauquoy, D.; Van Geel, B. Plant macrofossil methods and studies: Mire and peat macros. In Encyclopedia of Quaternary Science; Elsevier Science: Amsterdam, The Netherlands, 2007; pp. 2315–2336. [Google Scholar]
- Hölzer, A. Die Torfmoose: Südwestdeutschlands und der Nachbargebiete; Weissdorn-Verlag: Jena, Germany, 2010. [Google Scholar]
- Laine, J.; Flatberg, K.I.; Harju, P.; Timonen, T.; Minkkinen, K.J.; Laine, A.; Tuittila, E.-S.; Vasander, H.T. Sphagnum Mosses: The Stars of European Mires; Department of Forest Sciences, University of Helsinki: Helsinki, Finland, 2018. [Google Scholar]
- Erdtman, G. The acetolysis method-a revised description. Sven Bot Tidskr 1960, 54, 516–564. [Google Scholar]
- Moore, P.; Webb, J.; Collinson, M. Pollen Analysis, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1991. [Google Scholar]
- Davis, B.A.; Zanon, M.; Collins, P.; Mauri, A.; Bakker, J.; Barboni, D.; Barthelmes, A.; Beaudouin, C.; Bjune, A.E.; Bozilova, E. The European modern pollen database (EMPD) project. Veg. Hist. Archaeobotany 2013, 22, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Salonen, J.S.; Korpela, M.; Williams, J.W.; Luoto, M. Machine-learning based reconstructions of primary and secondary climate variables from North American and European fossil pollen data. Sci. Rep. 2019, 9, 15805. [Google Scholar] [CrossRef]
- Juggins, S. Rioja: Analysis of Quaternary Science Data, R Package Version (0.9-21); Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://cran.r-project.org/package=rioja (accessed on 28 October 2020).
- Simpson, G.L. Analogue methods in palaeoecology: Using the ‘analogue’ package. J. Stat. Soft. 2007, 22, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Mazei, Y.A.; Chernyshov, V.A. Testate amoebae communities in the southern tundra and forest-tundra of Western Siberia. Biol. Bull. 2011, 38, 789–796. [Google Scholar] [CrossRef]
- Mazei, Y.A.; Tsyganov, A.N. Freshwater Testate Amoebae; KMK: Moscow, Russia, 2006. [Google Scholar]
- Tsyganov, A.; Babeshko, K.; Mazei, Y. A Guide to Testate Amoebae with the Keys to Genera; Publ. House PSU: Penza, Russia, 2016. [Google Scholar]
- Tsyganov, A.N.; Babeshko, K.V.; Novenko, E.Y.; Malysheva, E.A.; Payne, R.J.; Mazei, Y.A. Quantitative reconstruction of peatland hydrological regime with fossil testate amoebae communities. Russ. J. Ecol. 2017, 48, 135–142. [Google Scholar] [CrossRef]
- Bjerring, R.; Bécares, E.; Declerck, S.; Gross, E.M.; Hansson, L.-A.; Kairesalo, T.; Nykänen, M.; Halkiewicz, A.; Kornijów, R.; Conde-Porcuna, J.M.; et al. Subfossil Cladocera in relation to contemporary environmental variables in 54 Pan-European lakes. Freshw. Biol. 2009, 54, 2401–2417. [Google Scholar] [CrossRef]
- Bledzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kelly, M.; Adams, C.; Graves, A.; Jamieson, J.; Krokowski, J.; Lycett, E.; Murray-Bligh, J.; Pritchard, S.; Wilkins, C. The Trophic Diatom Index: A User’s Manual; Environment Agency: Bristol, UK, 2001. [Google Scholar]
- Khotinski, N.A. Holocene of the Northern Eurasia; Nauka: Moskow, Russia, 1977. [Google Scholar]
- Feurdean, A.; Vannière, B.; Finsinger, W.; Warren, D.; Connor, S.C.; Forrest, M.; Liakka, J.; Panait, A.; Werner, C.; Andric, M.; et al. Fire hazard modulation by long-term dynamics in land cover and dominant forest type in eastern and central Europe. Biogeosciences 2020, 17, 1213–1230. [Google Scholar] [CrossRef] [Green Version]
- Kupriyanov, D.; Novenko, E.Y. Reconstruction of the Holocene Dynamics of Forest Fires in the Central Part of Meshcherskaya Lowlands According to Antracological Analysis. Contemp. Probl. Ecol. 2019, 12, 204–212. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Tsyganov, A.N.; Volkova, E.M.; Babeshko, K.V.; Lavrent’ev, N.V.; Mazei, Y.A. Changes of vegetation and climate in the north-west of the Central Russian upland in the Holocene. Izv. Ross. Akad. Nauk Seriya Geogr. 2016. [Google Scholar] [CrossRef] [Green Version]
- Novenko, E.Y.; Tsyganov, A.N.; Pisarchuk, N.M.; Volkova, E.M.; Babeshko, K.V.; Kozlov, D.N.; Shilov, P.M.; Payne, R.J.; Mazei, Y.A.; Olchev, A.V. Forest history, peatland development and mid- to late Holocene environmental change in the southern taiga forest of central European Russia. Quat. Res. 2018, 89, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Novenko, E.Y.; Olchev, A.V.; Desherevskaya, O.; Zuganova, I.S. Paleoclimatic reconstructions for the south of Valdai Hills (European Russia) as paleo-analogs of possible regional vegetation changes under global warming. Environ. Res. Lett. 2009, 4, 045016. [Google Scholar] [CrossRef] [Green Version]
- Niinemets, E.; Saarse, L. Holocene vegetation and land-use dynamics of south-eastern Estonia. Quat. Int. 2009, 207, 104–116. [Google Scholar] [CrossRef]
- Heikkilä, M.; Seppä, H. Holocene climate dynamics in Latvia, eastern Baltic region: A pollen-based summer temperature reconstruction and regional comparison. Boreas 2010, 39, 705–719. [Google Scholar] [CrossRef]
- Zernitskaya, V.; Mikhailov, N. Evidence of early farming in the Holocene pollen spectra of Belarus. Quat. Int. 2009, 203, 91–104. [Google Scholar] [CrossRef]
- Gałka, M.; Tobolski, K.; Zawisza, E.; Goslar, T. Postglacial history of vegetation, human activity and lake-level changes at Jezioro Linówek in northeast Poland, based on multi-proxy data. Veg. Hist. Archaeobotany 2014, 23, 123–152. [Google Scholar] [CrossRef] [Green Version]
- Stivrins, N.; Kalnina, L.; Veski, S.; Zeimule, S. Local and regional Holocene vegetation dynamics at two sites in Eastern Latvia. Boreal Environ. Res. 2014, 9, 310–322. [Google Scholar]
- Kuosmanen, N.; Seppä, H.; Reitalu, T.; Alenius, T.; Bradshaw, R.H.; Clear, J.L.; Filimonova, L.; Kuznetsov, O.; Zaretskaya, N. Long-term forest composition and its drivers in taiga forest in NW Russia. Veg. Hist. Archaeobotany 2016, 25, 221–236. [Google Scholar] [CrossRef]
- Barhoumi, C.; Peyron, O.; Joannin, S.; Subetto, D.; Kryshen, A.; Drobyshev, I.; Girardin, M.P.; Brossier, B.; Paradis, L.; Pastor, T.; et al. Gradually increasing forest fire activity during the Holocene in the northern Ural region (Komi Republic, Russia). Holocene 2019, 29, 1906–1920. [Google Scholar] [CrossRef]
- Novenko, E.Y.; Mazei, N.G.; Kupriyanov, D.A.; Volkova, E.M.; Tsyganov, A.N. Holocene dynamics of vegetation and ecological conditions in the center of the East European Plain. Russ. J. Ecol. 2018, 49. [Google Scholar] [CrossRef]
- Mauri, A.; Davis, B.; Collins, P.; Kaplan, J. The climate of Europe during the Holocene: A gridded pollen-based reconstruction and its multi-proxy evaluation. Quat. Sci. Rev. 2015, 112, 109–127. [Google Scholar] [CrossRef]
- Kremenetski, K.; Borisova, O.; Zelikson, E. The Late Glacial and Holocene history of vegetation in the Moscow region. Paleontol. J. 2000, 34, S67–S74. [Google Scholar]
- Zernitskaya, V.; Stančikaitė, M.; Vlasov, B.; Šeirienė, V.; Kisielienė, D.; Gryguc, G.; Skipitytė, R. Vegetation pattern and sedimentation changes in the context of the Lateglacial climatic events: Case study of Staroje Lake (Eastern Belarus). Quat. Int. 2015, 386, 70–82. [Google Scholar] [CrossRef]
- Seppä, H.; Poska, A. Holocene annual mean temperature changes in Estonia and their relationship to solar insolation and atmospheric circulation patterns. Quat. Res. 2004, 61, 22–31. [Google Scholar] [CrossRef]
- Andreeva, Z.V.; Artemenko, I.I.; Bader, O.N. Epokha Bronzy Lesnoy Polosy SSSR; Bader, O.N., Kraynov, D.A., Kosarev, M.F., Eds.; Nauka: Moscow, Russia, 1987. [Google Scholar]
- Islanova, I.V. O lokalnykh gruppakh dyakovskikh pamyatnikov. Tver. Arkheologichesky Sb. 2002, 5, 451–460. [Google Scholar]
- Kuneš, P.; Svobodová-Svitavská, H.; Kolář, J.; Hajnalová, M.; Abraham, V.; Macek, M.; Tkáč, P.; Szabó, P. The origin of grasslands in the temperate forest zone of east-central Europe: Long-term legacy of climate and human impact. Quat. Sci. Rev. 2015, 116, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Smirnov, A.L.; Kuprianov, D.I. Novye paleoekologicheskiye dannye gorodishch rannego zheleznogo veka v Verkhovyakh Volgi. Tr. XXII Syezda Arkheologov Ross. 2020, in press. [Google Scholar]
- Smirnov, A.L.; Dobrovolskaya, M.V.; Menshikov, M.Y. Arkheologicheskiye razvedki v Penovskom rayone Tverskoy oblasti v 2018–2019 gg. Arkheologicheskiye Otkrytiya 2020, in press. [Google Scholar]
- Swinehart, A.L.; Parker, G.R. Palaeoecology and development of peatlands in Indiana. Am. Midl. Nat. 2000, 143, 267–297. [Google Scholar] [CrossRef]
- Gałka, M.; Tobolski, K.; Kołaczek, P. The Holocene decline of slender naiad (Najas flexilis (Willd.) Rostk. & WLE Schmidt) in NE Poland in the light of new palaeobotanical data. Acta Palaeobot. 2012, 52, 127–138. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski [Guidebook for Determination of Plant Communities in Poland]; Wydaw. Nauk. PWN: Warszawa, Poland, 2001. [Google Scholar]
- Karpińska-Kołaczek, M.; Stachowicz-Rybka, R.; Obidowicz, A.; Woszczyk, M.; Kołaczek, P. A lake-bog succession vs. climate changes from 13,300 to 5900 cal. BP in NE Poland in the light of palaeobotanical and geochemical proxies. Rev. Palaeobot. Palynol. 2016, 233, 199–215. [Google Scholar] [CrossRef]
- Coletta, P.; Pentecost, A.; Spiro, B. Stable isotopes in charophyte incrustations: Relationships with climate and water chemistry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2001, 173, 9–19. [Google Scholar] [CrossRef]
- Pentecost, A.; Andrews, J.E.; Dennis, P.F.; Marca-Bell, A.; Dennis, S. Charophyte growth in small temperate water bodies: Extreme isotopic disequilibrium and implications for the palaeoecology of shallow marl lakes. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 240, 389–404. [Google Scholar] [CrossRef]
- Zarzycki, K.; Trzcińska-Tacik, H.; Różański, W.; Szeląg, Z.; Wołek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plants of Poland. Biodiversity of Poland (Volume 2); W Szafer Institute of Botany (Polish Academy of Sciences): Kraków, Poland, 2002. [Google Scholar]
- Płóciennik, M.; Pawłowski, D.; Vilizzi, L.; Antczak-Orlewska, O. From oxbow to mire: Chironomidae and Cladocera as habitat palaeoindicators. Hydrobiologia 2020, 847, 3257–3275. [Google Scholar] [CrossRef]
- Amon, L.; Heinsalu, A.; Veski, S. Late glacial multiproxy evidence of vegetation development and environmental change at Solova, southeastern Estonia. Est. J. Earth Sci. 2010, 59, 151. [Google Scholar] [CrossRef]
- Veski, S.; Amon, L.; Heinsalu, A.; Reitalu, T.; Saarse, L.; Stivrins, N.; Vassiljev, J. Lateglacial vegetation dynamics in the eastern Baltic region between 14,500 and 11,400 cal yr BP: A complete record since the Bølling (GI-1e) to the Holocene. Quat. Sci. Rev. 2012, 40, 39–53. [Google Scholar] [CrossRef]
- Kołaczek, P.; Mirosław-Grabowska, J.; Karpińska-Kołaczek, M.; Stachowicz-Rybka, R. Regional and local changes inferred from lacustrine organic matter deposited between the Late Glacial and mid-Holocene in the Skaliska Basin (north-eastern Poland). Quat. Int. 2015, 388, 51–63. [Google Scholar] [CrossRef]
- Gałka, M. Pattern of plant succession from eutrophic lake to ombrotrophic bog in NE Poland over the last 9400 years based on high-resolution macrofossil analysis. Ann. Bot. Fenn. 2014, 51, 1–21. [Google Scholar]
- Pawłowski, D. Evolution of an Eemian lake based on Cladocera analysis (Konin area, Central Poland). Acta Geol. Pol. 2011, 61, 441–450. [Google Scholar]
- Hughes, P.D.M.; Barber, K.E. Contrasting pathways to ombrotrophy in three raised bogs from Ireland and Cumbria, England. Holocene 2004, 14, 65–77. [Google Scholar] [CrossRef]
- Van Breemen, N. How Sphagnum bogs down other plants. Trends Ecol. Evol. 1995, 10, 270–275. [Google Scholar] [CrossRef]
- Väliranta, M.; Salojärvi, N.; Vuorsalo, A.; Juutinen, S.; Korhola, A.; Luoto, M.; Tuittila, E.-S. Holocene fen–bog transitions, current status in Finland and future perspectives. Holocene 2017, 27, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Hughes, P.; Dumayne-Peaty, L. Testing theories of mire development using multiple successions at Crymlyn Bog, West Glamorgan, South Wales, UK. J. Ecol. 2002, 90, 456–471. [Google Scholar] [CrossRef]
- Coulson, J.; Butterfield, J. An investigation of the biotic factors determining the rates of plant decomposition on blanket bog. J. Ecol. 1978, 66, 631–650. [Google Scholar] [CrossRef]









| Laboratory Code | Depth (cm) | Material | Radiocarbon Date (14C yr BP) | Calibrated Age BP (95% Confidence Interval) |
|---|---|---|---|---|
| IGAN-AMS-6596 | 52–53 | Sphagnum stems | 170 ± 20 | 1663–1694 AD (17.8%) |
| 1724–1785 AD (42.1%) | ||||
| 1794–1811 AD (9.7%) | ||||
| 1837–1844 AD (1%) | ||||
| 1851–1856 AD (0.7%) | ||||
| 1860–1866AD (0.7%) | ||||
| 1871–1877 AD (1%) | ||||
| 1915–1954 AD (22.1%) | ||||
| IGAN-AMS-6597 | 121–122 | Sphagnum stems | 580 ± 20 | 1310–1314 AD (1.3%) |
| 1317–1360 AD (66.5%) | ||||
| 1387–1409 AD (27.2%) | ||||
| IGAN-AMS-6598 | 215–216 | Sphagnum stems | 1190 ± 20 | 774–792 AD (16.1%) |
| 795–888 AD (78.9%) | ||||
| IGAN-AMS-6599 | 316–317 | Scheuchzeria sp. Rhizome | 1760 ± 20 | 238–265 AD (20.7%) |
| 272–350 AD (74%) | ||||
| 357–358 AD (0.3%) | ||||
| IGAN-AMS-6600 | 401–402 | Drepanocladus sp. remains | 2400 ± 20 | 714–712 BC (0.3%) |
| 659–656 BC (0.7%) | ||||
| 540–401 BC (93.9%) | ||||
| IGAN-AMS-6601 | 585–586 | Bark and wood of Populus sp. | 6040 ± 25 | 5008–4842 BC (95%) |
| IGAN-AMS-8002 | 589–590 | Unidentified plant remains | 6375 ± 25 | 5469–5439 BC (10.2%) |
| 5385–5304 BC (79.8%) | ||||
| 5249.89–5226 BC (5.1%) | ||||
| IGAN-AMS-8003 | 699–700 | Unidentified plant remains | 9160 ± 30 | 8530–8520 BC (2%) |
| 8460–8288 BC (93%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazei, Y.A.; Tsyganov, A.N.; Bobrovsky, M.V.; Mazei, N.G.; Kupriyanov, D.A.; Gałka, M.; Rostanets, D.V.; Khazanova, K.P.; Stoiko, T.G.; Pastukhova, Y.A.; et al. Peatland Development, Vegetation History, Climate Change and Human Activity in the Valdai Uplands (Central European Russia) during the Holocene: A Multi-Proxy Palaeoecological Study. Diversity 2020, 12, 462. https://0-doi-org.brum.beds.ac.uk/10.3390/d12120462
Mazei YA, Tsyganov AN, Bobrovsky MV, Mazei NG, Kupriyanov DA, Gałka M, Rostanets DV, Khazanova KP, Stoiko TG, Pastukhova YA, et al. Peatland Development, Vegetation History, Climate Change and Human Activity in the Valdai Uplands (Central European Russia) during the Holocene: A Multi-Proxy Palaeoecological Study. Diversity. 2020; 12(12):462. https://0-doi-org.brum.beds.ac.uk/10.3390/d12120462
Chicago/Turabian StyleMazei, Yuri A., Andrey N. Tsyganov, Maxim V. Bobrovsky, Natalia G. Mazei, Dmitry A. Kupriyanov, Mariusz Gałka, Dmitry V. Rostanets, Kseniya P. Khazanova, Tamara G. Stoiko, Yulia A. Pastukhova, and et al. 2020. "Peatland Development, Vegetation History, Climate Change and Human Activity in the Valdai Uplands (Central European Russia) during the Holocene: A Multi-Proxy Palaeoecological Study" Diversity 12, no. 12: 462. https://0-doi-org.brum.beds.ac.uk/10.3390/d12120462