Epilithic Diatom Community Shows a Higher Vulnerability of the River Sava to Pollution during the Winter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
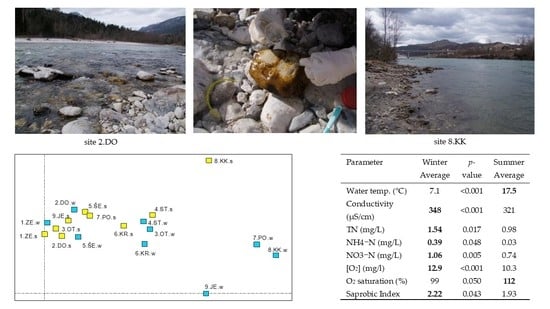
2.2. Sampling Sites
2.3. Measurements of Abiotic Parameters
2.4. Periphyton Sampling and Laboratory Analyses
2.5. Data Analysis
3. Results
3.1. Influence of Environmental Parametres on the Composition of the Diatom Community
3.2. Water Quality along the Course and between the Seasons
3.3. The Structure of the Diatom Community
Ecological Groups of Diatoms
4. Discussion
4.1. Influence of Environmental Parametres on the Composition of Epilithic Diatom Community
4.2. Water Quality along the Course and between the Seasons
Differences between Winter and Summer
4.3. The Structure of the Diatom Community
4.3.1. The Dominance of Taxa
4.3.2. Ecological Groups
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | Max. Velocity (m/s) | T Water (°C) | pH | Conductivity (µS/cm) | [O2] | Sat. with O2 (%) | TDS | PO4–P | TN | NH4–N | NO3–N |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZE.w | 0.76 | 4.8 | 7.2 | 262 | 10.4 | 83 | 258 | 0.03 | 1.11 | 0.03 | 0.86 |
| ZE.s | 1.20 | 8.1 | 7.1 | 254 | 8.0 | 79 | 250 | 0.00 | 0.79 | 0.02 | 0.31 |
| DO.w | 1.70 | 7.2 | 7.8 | 278 | 12.0 | 98 | 273 | 0.23 | 0.59 | 0.03 | 0.42 |
| DO.s | 1.74 | 10.2 | 7.5 | 263 | 9.9 | 95 | 256 | 0.00 | 0.35 | 0.02 | 0.44 |
| OT.w | 1.33 | 6.5 | 8.0 | 310 | 14.3 | 111 | 304 | 0.00 | 1.12 | 0.03 | 0.72 |
| OT.s | 1.39 | 16.5 | 8.0 | 280 | 10.9 | 117 | 276 | 0.14 | 0.86 | 0.03 | 0.71 |
| ST.w | 1.38 | 6.9 | 8.0 | 327 | 14.7 | 114 | 321 | 0.76 | 0.92 | 0.02 | 0.72 |
| ST.s | 1.53 | 18.3 | 8.0 | 300 | 10.0 | 110 | 297 | 0.01 | 0.84 | 0.03 | 0.55 |
| ŠE.w | 0.93 | 7.9 | 7.5 | 376 | 13.1 | 100 | 356 | 0.01 | 1.46 | 0.04 | 1.26 |
| ŠE.s | 0.37 | 17.2 | 7.6 | 340 | 10.1 | 104 | 329 | 0.00 | 1.09 | 0.04 | 0.81 |
| KR.w | 0.87 | 7.2 | 7.5 | 392 | 13.0 | 98 | 385 | 0.14 | 3.13 | 0.08 | 1.59 |
| KR.s | 0.83 | 18.5 | 7.8 | 363 | 10.7 | 116 | 357 | 0.03 | 1.44 | 0.06 | 1.15 |
| PO.w | 0.94 | 7.2 | 7.5 | 392 | 13.0 | 96 | 385 | 0.01 | 1.41 | 0.08 | 1.16 |
| PO.s | 0.85 | 20.1 | 7.9 | 370 | 11.0 | 123 | 365 | 0.06 | 1.38 | 0.05 | 1.00 |
| KK.w | 0.15 | 8.1 | 7.6 | 391 | 13.0 | 96 | 385 | 0.53 | 1.76 | 0.05 | 1.41 |
| KK.s | 0.04 | 22.3 | 8.1 | 359 | 11.7 | 133 | 354 | 0.01 | 0.72 | 0.02 | 0.73 |
| JE.w | 0.06 | 8.5 | 7.5 | 406 | 13.0 | 99 | 398 | 0.20 | 2.35 | 0.04 | 1.43 |
| JE.s | 0.59 | 26.3 | 8.1 | 363 | 10.7 | 132 | 357 | 0.23 | 1.38 | 0.04 | 0.99 |
References
- Dodds, W.K.; Biggs, B.J.F. Water Velocity Attenuation by Stream Periphyton and Macrophytes in Relation to Growth Form and Architecture. J. N. Am. Benthol. Soc. 2002, 21, 2–15. [Google Scholar] [CrossRef] [Green Version]
- Allan, J.D.; Castillo, M.M. Stream Ecology; Springer: Dordrecht, The Netherlands, 2007; p. 372. [Google Scholar]
- Izagirre, O.; Elosegi, A. Environmental control of seasonal and inter-annual variations of periphytic biomass in a North Iberian stream. Ann. Limnol. Int. J. Limnol. 2005, 41, 35–46. [Google Scholar] [CrossRef]
- Hill, W.R.; Ryon, M.G.; Smith, J.G.; Adams, S.M.; Boston, H.L.; Stewart, A.J. The Role of Periphyton in Mediating the Effects of Pollution in a Stream Ecosystem. Environ. Manag. 2010, 45, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Sabater, S.; Guasch, H.; Ricart, M.; Romaní, A.; Vidal, G.; Klünder, C.; Schmitt-Jansen, M. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Anal. Bioanal. Chem. 2007, 387, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Passy, S.I. Diatom ecological guilds display distinct and predictable behavior along nutrient and disturbance gradients in running waters. Aquat. Bot. 2007, 86, 171–178. [Google Scholar] [CrossRef]
- Toman, J.M.; Grošelj, A.M.; Zelnik, I. The Influence of Selected Factors on the Distribution of Epilithic Diatoms in a Torrential River the Kamniška Bistrica (Slovenia). Acta Bot. Croat. 2014, 73, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, R.J. Epilithic and epipelic diatoms in the Sandusky River, with emphasis on species diversity and water pollution. Hydrobiologia 1984, 114, 161–175. [Google Scholar] [CrossRef]
- Soininen, J.; Eloranta, P. Seasonal persistence and stability of diatom communities in rivers: Are there habitat specific differences? Eur. J. Phycol. 2004, 39, 153–160. [Google Scholar] [CrossRef]
- Gamier, J.; Billen, G.; Coste, M. Seasonal succession of diatoms and Chlorophyceae in the drainage network of the Seine River: Observation and modeling. Limnol. Oceanogr. 1995, 40, 750–765. [Google Scholar] [CrossRef]
- Soininen, J. Environmental and spatial control of freshwater diatoms—A review. Diatom Res. 2007, 22, 473–490. [Google Scholar] [CrossRef]
- Hofmann, G.; Werum, M.; Lange-Bertalot, H. Diatomeen im Süßwasser-Benthos von Mitteleuropa: Bestimmungsflora Kieselalgen für die Ökologische Praxis; Koeltz Scientific Books: Königstein, Germany, 2013; p. 908. [Google Scholar]
- Virtanen, L.K.; Köngäs, P.; Aitto-Oja, S.; Soininen, J. Is temporal occurrence of diatoms related to species traits, local abundance, and regional distribution? J. Phycol. 2011, 47, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Law, R.J.; Elliott, J.A.; Thackeray, S.J. Do functional or morphological classifications explain stream phytobenthic community assemblages? Diatom Res. 2014, 29, 309–324. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The stream and its valley. SIL Proc. 1922–2010 1975, 19, 1–15. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Reiners, W.A. Ecosystem Succession and Nutrient Retention: A Hypothesis. Bioscience 1975, 25, 376–381. [Google Scholar] [CrossRef]
- Valett, H.M.; Crenshaw, C.L.; Wagner, P.F. Stream nutrient uptake, forest succession, and biogeochemical theory. Ecology 2002, 83, 2888–2901. [Google Scholar] [CrossRef]
- Parr, L.B.; Mason, C.F. Long-term trends in water quality and their impact on macroinvertebrate assemblages in eutrophic lowland rivers. Water Res. 2003, 37, 2969–2979. [Google Scholar] [CrossRef]
- Bouletreau, S.; Garabetian, F.; Sauvage, S.; Sanchez-Perez, J.-M. Assessing the importance of a self-generated detachment process in river biofilm models. Freshw. Biol. 2006, 51, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Tuchman, N.C. The Role of Heterotrophy in Algae. Algal Ecol. 1996, 19, 299–319. [Google Scholar] [CrossRef]
- Frost, P.C.; Elser, J.J. Effects of light and nutrients on the net accumulation and elemental composition of epilithon in boreal lakes. Freshw. Biol. 2002, 47, 173–183. [Google Scholar] [CrossRef]
- Quinn, J.M.; Williamson, R.B.; Smith, R.K.; Vickers, M.L. Effects of riparian grazing and channelisation on streams in Southland, New Zealand. 2. Benthic invertebrates. N. Z. J. Mar. Freshw. Res. 1992, 26, 259–273. [Google Scholar] [CrossRef] [Green Version]
- Xenopoulos, M.A.; Schindler, D.W. Physical Factors Determining Ultraviolet Radiation Flux into Ecosystems. Ecosyst. Evol. Ultrav. Radiat. 2001, 36–62. [Google Scholar] [CrossRef]
- Kelly, D.J.; Clare, J.J.; Bothwell, M.L. Attenuation of solar ultraviolet radiation by dissolved organic matter alters benthic colonization patterns in streams. J. N. Am. Benthol. Soc. 2001, 20, 96–108. [Google Scholar] [CrossRef]
- Directive 2000/60/EC of the European Parlament and of the Council of 23 October 2000. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj (accessed on 8 October 2020).
- Rimet, F.; Bouchez, A. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl. Manag. Aquat. Ecosyst. 2012, 1–12. [Google Scholar] [CrossRef]
- Mataruga, Z.; Jarić, S.; Kostić, O.; Marković, M.; Jakovljević, K.; Mitrović, M.; Pavlović, P. The potential of elm trees (Ulmus glabra Huds.) for the phytostabilisation of potentially toxic elements in the riparian zone of the Sava River. Environ. Sci. Pollut. Res. 2020, 27, 4309–4324. [Google Scholar] [CrossRef]
- Rakocevic, J. Application of epilithic diatoms in the ecological assessment of mountain rivers: Contribution to the development of biomonitoring tools for Montenegrin aquatic ecosystems using the case study of the Tara River. Nova Hedwig. 2018, 106, 337–356. [Google Scholar] [CrossRef]
- Odum, E.P. Fundamentals of Ecology; W.B. Saunders Company: Philadelphia, PA, USA, 1971; p. 574. [Google Scholar]
- Udovičić, B. Energija i Okolina; Građevinska Knjiga: Beograd, Serbia, 1989; p. 190. [Google Scholar]
- Bricelj, M. River and Man—Sava, 1st ed.; Državna založba Slovenije: Ljubljana, Slovenia, 1991; p. 83. [Google Scholar]
- Biggs, B.J.F.; Kilroy, C. Stream Periphyton Monitoring Manual for The New Zealand Ministry for the Environment; National Institute of Water and Atmospheric Research: Christchurch, New Zealand, 2000; p. 246. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae; VEB G. Fischer. Jena: Jena, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae; VEB G. Fischer: Jena, Germany, 1988; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae—Teil 3: Centrales, Fragilariaceae, Eunotiaceae; VEB G. Fischer: Jena, Germany, 1991; p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 4. Teil: Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis; VEB G. Fischer: Jena, Germany, 1991; p. 437. [Google Scholar]
- Rott, E.; Pipp, E.; Pfister, P.; Van Dahm, H.; Ortler, K.; Binder, N.; Pall, K. Indikationslisten fur Aufwuchsalgen in Östereichen Fließgevessern, Teil 2: Trophienindikation so vie Geochemische Präferenz, Taxonomische und Toxicologische Anmerkungen; Bundesministerium für Land und Forstwirtschaft: Wien, Australia, 1999; p. 248.
- Kosi, G.; Šiško, M.; Bricelj, M.; Urbanič, G.; Grbović, J.; Rotar, B.; Stanič, D. Adaptation of Saprobic Index to the demands of Water Framework Directive (Directive 2000/60/EC) for the Assessment of Ecological State of the Rivers in Slovenia using Phytobenthos (in Slovenian); Environmental Agency of the Republic of Slovenia: Ljubljana, Slovenia, 2006; pp. 1–83.
- Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Cantonati, M. Diatom communities of springs in the southern Alps. Diatom Res. 1998, 13, 201–220. [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Ecology of Running Waters; University of Toronto Press: Toronto, ON, Canada, 1970; p. 555. [Google Scholar]
- Vermaat, J.E.; Hootsmans, M.J.M. Periphyton dynamics in a temperature-light gradient. In Lake Veluwe, a Macrophyte-Dominated System under Eutrophication Stress; Springer: Dordrecht, The Netherlands, 1994; pp. 193–212. [Google Scholar]
- Cole, J.J.; Caraco, N.F. Carbon in catchments: Connecting terrestrial carbon losses with aquatic metabolism. Mar. Freshw. Res. 2001, 52, 101. [Google Scholar] [CrossRef] [Green Version]
- Sládeček, V. System of Water Quality. Acta Hydrochim. Hydrobiol. 1973, 218. [Google Scholar] [CrossRef]
- Šaulys, V.; Survilė, O.; Stankevičienė, R. An Assessment of Self-Purification in Streams. Water 2020, 12, 87. [Google Scholar] [CrossRef] [Green Version]
- Eliasz-Kowalska, M.; Wojtal, A.Z. Limnological Characteristics and Diatom Dominants in Lakes of Northeastern Poland. Diversity 2020, 12, 374. [Google Scholar] [CrossRef]
- Exner-Kittridge, M.; Strauss, P.; Blöschl, G.; Eder, A.; Saracevic, E.; Zessner, M. The seasonal dynamics of the stream sources and input flow paths of water and nitrogen of an Austrian headwater agricultural catchment. Sci. Total Environ. 2016, 542, 935–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, R.T.; Ahmadi, M. Spatial and temporal variability of in-stream water quality parameter influence on dissolved oxygen and nitrate within a regional stream network. Ecol. Modell. 2014, 277, 87–96. [Google Scholar] [CrossRef]
- Webster, J.R.; Benfield, E.F.; Ehrman, T.P.; Schaeffer, M.A.; Tank, J.L.; Hutchens, J.J.; D’Angelo, D.J. What happens to allochthonous material that falls into streams? A synthesis of new and published information from Coweeta. Freshw. Biol. 1999, 41, 687–705. [Google Scholar] [CrossRef] [Green Version]
- Chénier, M.R.; Beaumier, D.; Roy, R.; Driscoll, B.T.; Lawrence, J.R.; Greer, C.W. Impact of Seasonal Variations and Nutrient Inputs on Nitrogen Cycling and Degradation of Hexadecane by Replicated River Biofilms. Appl. Environ. Microbiol. 2003, 69, 5170–5177. [Google Scholar] [CrossRef] [Green Version]
- Biggs, B.J.F.; Smith, R.A.; Duncan, M.J. Velocity and sediment disturbance of periphyton in headwater streams: Biomass and metabolism. J. N. Am. Benthol. Soc. 1999, 18, 222–241. [Google Scholar] [CrossRef]
- Cunha de Menezes, B.; Paulo, J.; Bittencourt, P.; Sá Farias, D.; Paulo Cunha de Menezes, J.; Parreira Bittencourt, R.; De Sá Farias, M.; Pinheiro Bello, I. Universidade de Taubaté Deoxygenation rate, reaeration and potential for self-purification of a small tropical urban stream. An. Interdiscip. J. Appl. Sci. 2015, 10, 748–757. [Google Scholar] [CrossRef]
- Rimet, F.; Cauchie, H.M.; Hoffmann, L.; Ector, L. Response of diatom indices to simulated water quality improvements in a river. J. Appl. Phycol. 2005, 17, 119–128. [Google Scholar] [CrossRef]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessment; Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; p. 942. [Google Scholar]
- Rakowska, B.; Szczepocka, E. Demonstration of the Bzura River restoration using diatom indices. Biologia 2011, 66, 411–417. [Google Scholar] [CrossRef]
- Menegalija, T.; Kosi, G. Distribution of diatoms in springs in the Julian Alps (NW Slovenia). Nat. Slov. 2008, 10, 21–37. [Google Scholar]
- Beauger, A.; Allain, E.; Voldoire, O.; Wetzel, C.E.; Ector, L.; Van de Vijver, B. Temporal Evolution of Diatoms in a Temporary Pond Situated in the Massif du Sancy Mountains (Massif Central, France) and Description of a New Pinnularia Species. Diversity 2020, 12, 367. [Google Scholar] [CrossRef]
- Biggs, B.J.F.; Goring, D.G.; Nikora, V.I. Subsidy and stress responses of stream periphyton to gradients in water velocity as a function of community growth form. J. Phycol. 1998, 34, 598–607. [Google Scholar] [CrossRef]
- Hondzo, M.; Wang, H. Effects of turbulence on growth and metabolism of periphyton in a laboratory flume. Water Resour. Res. 2002, 38, 13-1–13-9. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Zelnik, I.; Balanč, T.; Toman, M. Diversity and Structure of the Tychoplankton Diatom Community in the Limnocrene Spring Zelenci (Slovenia) in Relation to Environmental Factors. Water 2018, 10, 361. [Google Scholar] [CrossRef] [Green Version]
- Battegazzore, M.; Pompilio, L.; Botta, P.; D’Arnese, L.; Benigni, E.; Bertola, A.; Spano, M. Diatoms used for the evaluation of the effects of experimental water releases from hydroelectric power plants in alpine river systems: The case of the Cairasca-Devero basins (NW Italy). In Proceedings of the 7th CE-Diatom Meeting, Thonon-les-Bains, France, 16–20 September 2013; pp. 108–110. [Google Scholar]
- Gallo, L.; Battegazzore, M.; Corapi, A.; de Filippis, A.; Mezzotero, A.; Lucadamo, L. Environmental analysis of a regulated Mediterranean stream based on epilithic diatom communities—The Crati River case (southern Italy). Diatom Res. 2013, 28, 143–156. [Google Scholar] [CrossRef]








| Quality Index | Ecological Groups | |||||
|---|---|---|---|---|---|---|
| TI | SI | LP | HP | M | PL | |
| Altitude | −0.660 | −0.784 | −0.552 | |||
| Distance from the source | 0.660 | 0.784 | 0.552 | |||
| Channel width | 0.776 | 0.743 | −0.554 | 0.659 | ||
| Periphyton dry mass | 0.550 | |||||
| Current velocity | −0.546 | |||||
| T water | −0.593 | |||||
| Conductivity | 0.717 | 0.792 | 0.676 | |||
| PO4–P | 0.533 | 0.497 | ||||
| TDS | 0.771 | 0.839 | −0.473 | 0.721 | ||
| TN | 0.530 | 0.548 | 0.604 | |||
| NH4–N | 0.520 | 0.551 | 0.481 | |||
| NO3–N | 0.589 | 0.638 | 0.611 | |||
| Winter Samples Average | p-Value | Summer Samples Average | |
|---|---|---|---|
| Parameter: | |||
| Water temperature (°C) | 7.1 | <0.001 | 17.5 |
| Conductivity (µS/cm) | 348 | <0.001 | 321 |
| TDS (mg/L) | 341 | <0.001 | 316 |
| TN (mg/L) | 1.54 | 0.017 | 0.98 |
| NH4–N (mg/L) | 0.39 | 0.048 | 0.03 |
| NO3––N (mg/L) | 1.06 | 0.005 | 0.74 |
| [O2] (mg/L) | 12.9 | <0.001 | 10.3 |
| O2 saturation (%) | 99 | 0.050 | 112 |
| SI | 2.22 | 0.043 | 1.93 |
| Species number | 36.1 | 0.003 | 26.6 |
| Shannon diversity index | 3.70 | 0.012 | 2.94 |
| ZE | DO | OT | ST | ŠE | KR | PO | KK | JE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxa | w | s | w | s | w | s | w | s | w | s | w | s | w | s | w | s | w | s |
| Achnanthidium minutissimum | 41 | 51 | 30 | 48 | 23 | 41 | 22 | 38 | 30 | 26 | 16 | 27 | 16 | 36 | ||||
| Achnanthidium pyrenaicum | 20 | 21 | 25 | 35 | 13 | 38 | 12 | 37 | 29 | 14 | 17 | 24 | 42 | |||||
| Encyonema minutum | 15 | 10 | 17 | |||||||||||||||
| Gomphonema tergestinum | 15 | 32 | ||||||||||||||||
| Navicula lanceolata | 17 | 27 | ||||||||||||||||
| Nitzschia fonticola | 15 | 13 | ||||||||||||||||
| Cyclotella meneghiniana | 42 | |||||||||||||||||
| Amphora pediculus | 20 | |||||||||||||||||
| Surirella minuta | 11 | |||||||||||||||||
| Navicula reichardtiana | 7 | |||||||||||||||||
| Cocconeis placentula | 17 | |||||||||||||||||
| Diatoma ehrenbergii | 13 | |||||||||||||||||
| Mayamaea atomus | 10 | |||||||||||||||||
| Nitzschia gracilis | 10 | |||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelnik, I.; Sušin, T. Epilithic Diatom Community Shows a Higher Vulnerability of the River Sava to Pollution during the Winter. Diversity 2020, 12, 465. https://0-doi-org.brum.beds.ac.uk/10.3390/d12120465
Zelnik I, Sušin T. Epilithic Diatom Community Shows a Higher Vulnerability of the River Sava to Pollution during the Winter. Diversity. 2020; 12(12):465. https://0-doi-org.brum.beds.ac.uk/10.3390/d12120465
Chicago/Turabian StyleZelnik, Igor, and Tjaša Sušin. 2020. "Epilithic Diatom Community Shows a Higher Vulnerability of the River Sava to Pollution during the Winter" Diversity 12, no. 12: 465. https://0-doi-org.brum.beds.ac.uk/10.3390/d12120465