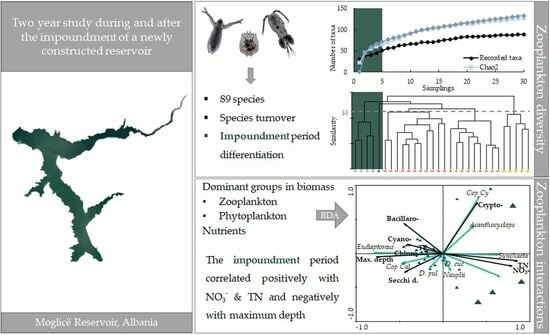
Pelagial Zooplankton Community in a Newly Established Reservoir during and after the Impoundment of a Hydropower Dam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samplings
2.3. Sample Analysis
2.4. Data Analysis
3. Results
3.1. Species Composition
3.2. Abundance-Biomass
3.3. Zooplankton Relations with Phytoplankton and Environmental Variables
3.3.1. Environmental Parameters
3.3.2. Phytoplankton Community
3.3.3. Redundancy Analysis
3.3.4. Grazing Potential Index
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stickler, C.M.; Coe, M.T.; Costa, M.H.; Nepstad, D.C.; McGrath, D.G.; Dias, L.C.P.; Rodrigues, H.O.; Soares-Filho, B.S. Dependence of Hydropower Energy Generation on Forests in the Amazon Basin at Local and Regional Scales. Proc. Natl. Acad. Sci. USA 2013, 110, 9601–9606. [Google Scholar] [CrossRef]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H. Mapping the World’s Free-Flowing Rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- World Commission on Dams Dams and Development: A New Framework for Decision-Making. Environ. Manag. Health 2001, 12, 444–445. [CrossRef]
- Kornijów, R. Controversies around Dam Reservoirs: Benefits, Costs and Future. Ecohydrol. Hydrobiol. 2009, 9, 141–148. [Google Scholar] [CrossRef]
- Bartle, A. Hydropower Potential and Development Activities. Energy Policy 2002, 30, 1231–1239. [Google Scholar] [CrossRef]
- Zohary, T.; Ostrovsky, I. Ecological Impacts of Excessive Water Level Fluctuations in Stratified Freshwater Lakes. Inland Waters 2011, 1, 47–59. [Google Scholar] [CrossRef]
- García, A.; Jorde, K.; Habit, E.; Caamaño, D.; Parra, O. Downstream Environmental Effects of Dam Operations: Changes in Habitat Quality for Native Fish Species. River Res. Appl. 2011, 27, 312–327. [Google Scholar] [CrossRef]
- Morita, K.; Morita, S.H.; Yamamoto, S. Effects of Habitat Fragmentation by Damming on Salmonid Fishes: Lessons from White-Spotted Charr in Japan. Ecol. Res. 2009, 24, 711–722. [Google Scholar] [CrossRef]
- Tonkin, J.D.; Death, R.G.; Joy, M.K. Invertebrate Drift Patterns in a Regulated River: Dams, Periphyton Biomass or Longitudinal Patterns? River Res. Appl. 2009, 25, 1219–1231. [Google Scholar] [CrossRef]
- Zhou, S.; Tang, T.; Wu, N.; Fu, X.; Jiang, W.; Li, F.; Cai, Q. Impacts of Cascaded Small Hydropower Plants on Microzooplankton in Xiangxi River, China. Acta Ecol. Sin. 2009, 29, 62–68. [Google Scholar] [CrossRef]
- de Souza, C.A.; Vieira, L.C.G.; Legendre, P.; de Carvalho, P.; Velho, L.F.M.; Beisner, B.E. Damming Interacts with the Flood Pulse to Alter Zooplankton Communities in an Amazonian River. Freshw. Biol. 2019, 64, 1040–1053. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as Indicators in Lakes: A Scientific-Based Plea for Including Zooplankton in the Ecological Quality Assessment of Lakes According to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Sterner, R.W. Role of Zooplankton in Aquatic Ecosystems. In Encyclopedia of Inland Waters; Elsevier Inc.: Amsterdam, The Netherlands, 2009; pp. 678–688. ISBN 978-0-12-370626-3. [Google Scholar]
- Hébert, M.-P.; Beisner, B.E.; Maranger, R. Linking Zooplankton Communities to Ecosystem Functioning: Toward an Effect-Trait Framework. J. Plankton Res. 2017, 39, 3–12. [Google Scholar] [CrossRef]
- Caroni, R.; Irvine, K. The Potential of Zooplankton Communities for Ecological Assessment of Lakes: Redundant Concept or Political Oversight? Biol. Environ. Proc. R. Ir. Acad. 2010, 110, 35–53. [Google Scholar] [CrossRef]
- Lampert, W.; Fleckner, W.; Rai, H.; Taylor, B.E. Phytoplankton Control by Grazing Zooplankton: A Study on the Spring Clear-Water Phase1. Limnol. Oceanogr. 1986, 31, 478–490. [Google Scholar] [CrossRef]
- Lathrop, R.C.; Carpenter, S.R.; Robertson, D.M. Summer Water Clarity Responses to Phosphorus, Daphnia Grazing, and Internal Mixing in Lake Mendota. Limnol. Oceanogr. 1999, 44, 137–146. [Google Scholar] [CrossRef]
- Hessen, D.O. Nutrient Element Limitation of Zooplankton Production. Am. Nat. 1992, 140, 799–814. [Google Scholar] [CrossRef]
- Elser, J.J.; Goldman, C.R. Zooplankton Effects on Phytoplankton in Lakes of Contrasting Trophic Status. Limnol. Oceanogr. 1991, 36, 64–90. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Carlson, C.A.; Bates, N.R.; Goldthwait, S.A.; Madin, L.P.; Michaels, A.F. Zooplankton Vertical Migration and the Active Transport of Dissolved Organic and Inorganic Carbon in the Sargasso Sea. Deep Sea Res. Part Oceanogr. Res. 2000, 47, 137–158. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-Model of Seasonal Succession of Planktonic Events in Fresh Waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Michaloudi, E.; Sommer, U. Modifying the PEG Model for Mediterranean Lakes—No Biological Winter and Strong Fish Predation. Freshw. Biol. 2014, 59, 1136–1144. [Google Scholar] [CrossRef]
- Paquette, C.; Gregory-Eaves, I.; Beisner, B.E. Environmental Drivers of Taxonomic and Functional Variation in Zooplankton Diversity and Composition in Freshwater Lakes across Canadian Continental Watersheds. Limnol. Oceanogr. 2022, 67, 1081–1097. [Google Scholar] [CrossRef]
- Muñoz-Colmenares, M.E.; Vicente, E.; Soria, J.M.; Miracle, M.R. Zooplankton Changes at Six Reservoirs in the Ebro Watershed, Spain. Limnetica 2021, 40, 279–294. [Google Scholar] [CrossRef]
- Sampaio, E.V.; Rocha, O.; Matsumura-Tundisi, T.; Tundisi, J.G. Composition and Abundance of Zooplankton in the Limnetic Zone of Seven Reservoirs of the Paranapanema River, Brazil. Braz. J. Biol. 2002, 62, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Parkes, S.M.; Duggan, I.C. Are Zooplankton Invasions in Constructed Waters Facilitated by Simple Communities? Divers. Distrib. 2012, 18, 1199–1210. [Google Scholar] [CrossRef]
- Jenkins, D.G. Dispersal-Limited Zooplankton Distribution and Community Composition in New Ponds. Hydrobiologia 1995, 313, 15–20. [Google Scholar] [CrossRef]
- Frisch, D.; Cottenie, K.; Badosa, A.; Green, A.J. Strong Spatial Influence on Colonization Rates in a Pioneer Zooplankton Metacommunity. PLoS ONE 2012, 7, e40205. [Google Scholar] [CrossRef]
- Cohen, G.M.; Shurin, J.B. Scale-Dependence and Mechanisms of Dispersal in Freshwater Zooplankton. Oikos 2003, 103, 603–617. [Google Scholar] [CrossRef]
- Havel, J.E.; Shurin, J.B. Mechanisms, Effects, and Scales of Dispersal in Freshwater Zooplankton. Limnol. Oceanogr. 2004, 49, 1229–1238. [Google Scholar] [CrossRef]
- Statkraft Moglice Hydropower Plant. Available online: https://www.statkraft.com/about-statkraft/where-we-operate/albania/moglice-hydropower-plant/ (accessed on 29 December 2022).
- ESIA. Devoll Hydropower Project Engineering Services Development Phase; ESIA: Hawthorn, Australia, 2011; p. 58. [Google Scholar]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Trophic State Assessment Based on Zooplankton Communities in Mediterranean Lakes. Hydrobiologia 2019, 844, 83–103. [Google Scholar] [CrossRef]
- Jersabek, C.D.; Leitner, M.F. The Rotifer World Catalog. World Wide Web Electronic Publication. Available online: http://www.rotifera.hausdernatur.at/ (accessed on 29 December 2022).
- Segers, H.; De Smet, W.H.; Fischer, C.; Fontaneto, D.; Michaloudi, E.; Wallace, R.L.; Jersabek, C.D. Towards a List of Available Names in Zoology, Partim Phylum Rotifera. Zootaxa 2012, 3179, 61–68. [Google Scholar] [CrossRef]
- International Commission on Zoological Nomenclature Rotifer Parts of the LAN. Available online: https://www.iczn.org/list-of-available-names/rotifer-lan/ (accessed on 26 January 2023).
- Kotov, A.; Forró, L.; Korovchinsky, N.M.; Petrusek, A. World Checklist of Freshwater Cladocera Species. Available online: http://fada.biodiversity.be/group/show/17 (accessed on 17 December 2022).
- WoRMS Editorial Board World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 30 December 2022).
- Bottrell, H.C. A Review of Some Problems in Zooplankton Production Studies. Norw. J. Zool 1976, 24, 419–456. [Google Scholar]
- Downing, J.A.; Rigler, F.H. A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters; Blackwell Scientific Publications: Hoboken, NJ, USA, 1984; ISBN 978-0-632-00616-8. [Google Scholar]
- Taggart, C.T. Hypolimnetic Aeration and Zooplankton Distribution: A Possible Limitation to the Restoration of Cold-Water Fish Production. Can. J. Fish. Aquat. Sci. 1984, 41, 191–198. [Google Scholar] [CrossRef]
- Dumont, H.J.; Van de Velde, I.; Dumont, S. The Dry Weight Estimate of Biomass in a Selection of Cladocera, Copepoda and Rotifera from the Plankton, Periphyton and Benthos of Continental Waters. Oecologia 1975, 19, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Michaloudi, E. Dry Weights of the Zooplankton of Lake Mikri Prespa (Macedonia, Greece). Belg. J. Zool 2005, 135, 223–227. [Google Scholar]
- Haberman, J. An Ecological Characterization of the Rotifers Dominating in the Pelagic Region of Lakes Peipsi-Pihkva and Võrtsjärv. Product. Est. Fresh Waters Est. Contrib. Int. Biol. Programme 1976, 10, 35–59. [Google Scholar]
- Haberman, J. An Ecological Characterization of the Crustaceans (Cladocera, Copepoda) and Dreissena Polymorpha Juv. Dominating in the Pelagic Region of Lakes Peipsi-Pihkva and Võrtsjärv. Product. Est. Fresh Waters Est. Contrib. Int. Biol. Programme 1976, 10, 60–99. [Google Scholar]
- Utermöhl, H. Methods of Collecting Plankton for Various Purposes Are Discussed. SIL Commun. 1953–1996 1958, 9, 1–38. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Simões, N.R.; Nunes, A.H.; Dias, J.D.; Lansac-Tôha, F.A.; Velho, L.F.M.; Bonecker, C.C. Impact of Reservoirs on Zooplankton Diversity and Implications for the Conservation of Natural Aquatic Environments. Hydrobiologia 2015, 758, 3–17. [Google Scholar] [CrossRef]
- Colwell, R.K.; Michelotto, A.S.; Javier, S.C.C.; Toniotti, D.G.A. EstimateS_9.1_Windows 2022. Available online: osf.io/su57f (accessed on 15 December 2022).
- Clarke, K.R.; Gorley, R.N. Primer. Primer-E Plymouth 2006, 115. [Google Scholar]
- Baselga, A. Partitioning the Turnover and Nestedness Components of Beta Diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, D.; Villeger, S.; de Bortoli, J.; Lepieur, F.; Logez, M. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components. R Package Version 2022, 1, 6. [Google Scholar]
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Baselga, M.A. Package ‘Betapart’ 1.5.6. 2022. Available online: https://CRAN.R-project.org/package=betapart (accessed on 10 January 2023).
- R Core Team. A Language and Environment for Statistical Computing. 2022. Available online: https://www.r-project.org/ (accessed on 10 January 2023).
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); WUR: Wageningen, The Netherlands, 2002. [Google Scholar]
- Jeppesen, E.; Peder Jensen, J.; Søndergaard, M.; Lauridsen, T.; Junge Pedersen, L.; Jensen, L. Top-down Control in Freshwater Lakes: The Role of Nutrient State, Submerged Macrophytes and Water Depth. Hydrobiologia 1997, 342, 151–164. [Google Scholar] [CrossRef]
- Arp, W.; Deneke, R. Untersuchungen des Phyto- und Zooplanktons Schleswig-Holsteinischer Seen 2006; Im Auftrag des Landesamtes für Natur und Umwelt Schleswig-Holsteins, Abteilung: Berlin, Germany, 2007; p. 225. [Google Scholar]
- Arp, W.; Kasten, J.; Maier, G. Untersuchungen Des Phyto- Und Zooplanktons Schleswig-Holsteinischer Seen 2009; Im Auftrag des Landesamtes für Natur und Umwelt Schleswig-Holsteins, Abteilung: Berlin, Germany, 2010; p. 248. [Google Scholar]
- Kozhuharov, D. The Zooplankton from Some Lakes and Ponds in North Albania with Different Size and Altitude. Hist. Nat. Bulg. 2000, 11, 33–37. [Google Scholar]
- Shumka, S. Checklist of Rotifer Species from Albania (Phylum Rotifera). Opusc. Zool. Bp. 2021, 52, 99–109. [Google Scholar] [CrossRef]
- Michaloudi, E.; Zarfdjian, M.; Economidis, P.S. The Zooplankton of Lake Mikri Prespa. Hydrobiologia 1997, 361, 77–94. [Google Scholar] [CrossRef]
- Shehu, M.; Serravalle, F.; Alfonso, G.; Moscatello, S.; Belmonte, G. The Alpine Gistova (Mount Gramos, Albania-Greece Border) Biodiversity of an Isolated Microcosm. Thalass. Salentina 2009, 32, 53–62. [Google Scholar] [CrossRef]
- Stamou, G.; Savva, A.; Demertzioglou, M.; Michaloudi, E. Diversity of Rotifera (Subclass: Monogononta) from Inland Water Bodies in Greece: An Updated Checklist. Diversity 2022, 14, 451. [Google Scholar] [CrossRef]
- Stamou, G.; Kourkoutmani, P.; Michaloudi, E. The Inland Cladocera and Copepoda Fauna in Greece. Diversity 2022, 14, 997. [Google Scholar] [CrossRef]
- Michaloudi, E.; Papakostas, S.; Stamou, G.; Neděla, V.; Tihlaříková, E.; Zhang, W.; Declerck, S.A. Reverse Taxonomy Applied to the Brachionus Calyciflorus Cryptic Species Complex: Morphometric Analysis Confirms Species Delimitations Revealed by Molecular Phylogenetic Analysis and Allows the (Re) Description of Four Species. PLoS ONE 2018, 13, e0203168. [Google Scholar] [CrossRef]
- Morgan, N.C.; Backiel, T.; Bretschko, G.; Duncan, A.; Hillbricht-Ilkowska, A.; Kajak, Z.; Kitchell, J.F.; Larsson, P.; Levegue, C.; Nauwerck, A. Secondary Production. In The Functioning of Freshwater Ecosystems; Le Cren, E., Lowe-McConnell, R., Eds.; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Nogueira, M.G.; Reis Oliveira, P.C.; Tenorio de Britto, Y. Zooplankton Assemblages (Copepoda and Cladocera) in a Cascade of Reservoirs of a Large Tropical River (SE Brazil). Limnetica 2008, 27, 151–170. [Google Scholar] [CrossRef]
- Alfonso, G.; Belmonte, G.; Marrone, F.; Naselli-Flores, L. Does Lake Age Affect Zooplankton Diversity in Mediterranean Lakes and Reservoirs? A Case Study from Southern Italy. Hydrobiologia 2010, 653, 149–164. [Google Scholar] [CrossRef]
- Stage Sø, J.; Sand-Jensen, K.; Baastrup-Spohr, L. Temporal Development of Biodiversity of Macrophytes in Newly Established Lakes. Freshw. Biol. 2020, 65, 379–389. [Google Scholar] [CrossRef]
- Schmidt, J.; de Andrade, P.D.B.; Padial, A.A. Zooplankton Trajectory before, during and after a Hydropower Dam Construction. Acta Limnol. Bras. 2020, 32, e18. [Google Scholar] [CrossRef]
- Kramer, A.M.; Sarnelle, O.; Knapp, R.A. Allee Effect Limits Colonization Success of Sexually Reproducing Zooplankton. Ecology 2008, 89, 2760–2769. [Google Scholar] [CrossRef]
- Bledzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Moustaka-Gouni, M.; Alabanakis, K.; Mitrakas, M.; Psilovikos, A. Planktic Autotrophs and Environmental Conditions in the Newly-Formed Hydroelectric Thesaurus Reservoir, Greece. Arch. Hydrobiol. 2000, 149, 507–526. [Google Scholar] [CrossRef]
- Morais, P.; Chícharo, M.A.; Chícharo, L. Changes in a Temperate Estuary during the Filling of the Biggest European Dam. Sci. Total Environ. 2009, 407, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Ostrofsky, M.L. Trophic Changes in Reservoirs; an Hypothesis Using Phosphorus Budget Models. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1978, 63, 481–499. [Google Scholar] [CrossRef]
- Ostrofsky, M.L.; Duthie, H.C. An Approach to Modelling Productivity in Reservoirs: With 1 Figure in the Text. Int. Ver. Theor. Angew. Limnol. Verh. 1978, 20, 1562–1567. [Google Scholar] [CrossRef]
- Schallenberg, M.; Burns, C.W. Phytoplankton Biomass and Productivity in Two Oligotrophic Lakes of Short Hydraulic Residence Time. N. Z. J. Mar. Freshw. Res. 1997, 31, 119–134. [Google Scholar] [CrossRef]
- Gulati, R.; Demott, W. The Role of Food Quality for Zooplankton: Remarks on the State-of-the-Art, Perspectives and Priorities. Freshw. Biol. 1997, 38, 753–768. [Google Scholar] [CrossRef]
- Brooks, J.L.; Dodson, S.I. Predation, Body Size, and Composition of Plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef]
- Attayde, J.L.; Hansson, L.-A. The Relative Importance of Fish Predation and Excretion Effects on Planktonic Communities. Limnol. Oceanogr. 2001, 46, 1001–1012. [Google Scholar] [CrossRef]
- Drenner, R.W.; Smith, J.D.; Threlkeld, S.T. Lake Trophic State and the Limnological Effects of Omnivorous Fish. Hydrobiologia 1996, 319, 213–223. [Google Scholar] [CrossRef]
- Hansson, L.-A.; Nicolle, A.; Brodersen, J.; Romare, P.; Anders Nilsson, P.; Brönmark, C.; Skov, C. Consequences of Fish Predation, Migration, and Juvenile Ontogeny on Zooplankton Spring Dynamics. Limnol. Oceanogr. 2007, 52, 696–706. [Google Scholar] [CrossRef]
- Mehner, T.; Thiel, R. A Review of Predation Impact by 0+ Fish on Zooplankton in Fresh and Brackish Waters of the Temperate Northern Hemisphere. Environ. Biol. Fishes 1999, 56, 169–181. [Google Scholar] [CrossRef]
- Michaloudi, E.; Moustaka-Gouni, M.; Katsiapi, M.; Kokkinakis, A.; Stamou, G.; Bozatzidou, M.; Varveris, T. Biomonitoring and Ecological Water Quality of Devoll River-Reservoir System; Aristotle University of Thessaloniki: Thessaloniki, Greece, 2022; p. 134. [Google Scholar]
- Stamou, G.; Katsiapi, M.; Moustaka-Gouni, M.; Michaloudi, E. Grazing Potential—A Functional Plankton Food Web Metric for Ecological Water Quality Assessment in Mediterranean Lakes. Water 2019, 11, 1274. [Google Scholar] [CrossRef] [Green Version]









| Taxa | Habitat | Occurrence | F.O. | Taxa | Habitat | Occurrence | F.O. |
|---|---|---|---|---|---|---|---|
| Rotifera | |||||||
| Ascomorpha agilis Zacharias, 1893 | Pelagic | rare | 3.33 | Lecane bulla (Gosse, 1851) | Littoral | rare | 3.33 |
| Ascomorpha ecaudis Perty, 1850 | Pelagic | rare | 16.67 | Lecane closterocerca (Schmarda, 1859) | Littoral | accessory | 36.67 |
| Ascomorpha saltans Bartsch, 1870 | Pelagic | rare | 10.00 | Lecane flexilis (Gosse, 1886) | Littoral | accessory | 40.00 |
| Asplanchna girodi Guerne, 1888 | Pelagic | rare | 6.67 | Lecane hamata (Stokes, 1896) | Littoral | rare | 13.33 |
| Asplanchna priodonta Gosse, 1850 | Pelagic | constant | 56.67 | Lecane luna (Müller, 1776) | Littoral | rare | 3.33 |
| Bdelloidea Hudson, 1884 | Littoral | constant | 73.33 | Lecane lunaris (Ehrenberg, 1832) | Littoral | rare | 3.33 |
| Brachionus angularis Gosse, 1851 | Pelagic | rare | 16.67 | Lecane nana (Murray, 1913) | Littoral | rare | 3.33 |
| Brachionus fernandoi Michaloudi et al. 2018 | Pelagic | rare | 3.33 | Lepadella patella (Müller, 1773) | Littoral | rare | 6.67 |
| Brachionus leydigii Cohn, 1862 | Pelagic | rare | 3.33 | Lepadella quadricarinata (Stenroos, 1898) | Littoral | rare | 3.33 |
| Cephalodella gibba (Ehrenberg, 1830) | Littoral | accessory | 26.67 | Lepadella rhomboides (Gosse, 1886) | Littoral | accessory | 40.00 |
| Cephalodella forficula (Ehrenberg, 1838) | Littoral | rare | 6.67 | Lophocharis salpina (Ehrenberg, 1834) | Littoral | accessory | 36.67 |
| Collotheca sp. | Pelagic | constant | 56.67 | Monommata sp. | Littoral | rare | 6.67 |
| Colurella hindenburgi Steinecke, 1916 | Littoral | rare | 6.67 | Mytilina brevispina (Ehrenberg, 1830) | Littoral | rare | 3.33 |
| Colurella uncinata (Müller, 1773) | Littoral | rare | 3.33 | Mytilina mucronata (Müller, 1773) | Littoral | accessory | 20.00 |
| Conochilus dossuarius Hudson, 1885 | Pelagic | rare | 3.33 | Notholca acuminata (Ehrenberg, 1832) | Pelagic | rare | 13.33 |
| Conochilus unicornis Rousselet, 1892 | Pelagic | accessory | 46.67 | Notholca squamula (Müller, 1786) | Pelagic | rare | 10.00 |
| Dicranophoroides caudatus (Ehrenberg, 1834) | Littoral | rare | 6.67 | Platyias quadricornis (Ehrenberg, 1832) | Pelagic | rare | 3.33 |
| Dicranophorus forcipatus (Müller, 1786) | Littoral | rare | 3.33 | Polyarthra dolichoptera Idelson, 1925 | Pelagic | accessory | 30.00 |
| Encentrum sp. | Littoral | rare | 3.33 | Polyarthra luminosa Kutikova, 1962 | Pelagic | constant | 63.33 |
| Eothinia elongata (Ehrenberg, 1832) | Littoral | rare | 3.33 | Polyarthra major Burckhardt, 1900 | Pelagic | accessory | 26.67 |
| Epiphanes macroura (Barrois & Daday, 1894) | Pelagic | rare | 3.33 | Polyarthra remata Skorikov, 1896 | Pelagic | rare | 16.67 |
| Euchlanis dilatata lucksiana Hauer 1930 | Pelagic | rare | 6.67 | Pompholyx complanata Gosse, 1851 | Pelagic | constant | 73.33 |
| Filinia longiseta (Ehrenberg, 1834) | Pelagic | constant | 56.67 | Pompholyx sulcata Hudson, 1885 | Pelagic | rare | 16.67 |
| Filinia terminalis (Plate, 1886) | Pelagic | constant | 56.67 | Synchaeta spp. | Pelagic | constant | 70.00 |
| Gastropus stylifer Imhof, 1891 | Pelagic | rare | 10.00 | Testudinella mucronata (Gosse, 1886) | Pelagic | rare | 3.33 |
| Hexarthra mira (Hudson, 1871) | Pelagic | accessory | 23.33 | Testudinella patina (Hermann, 1783) | Pelagic | rare | 10.00 |
| Kellicottia longispina (Kellicott, 1879) | Pelagic | constant | 56.67 | Trichocerca capucina (Wierzejski & Zacharias, 1893) | Pelagic | rare | 3.33 |
| Keratella cochlearis (Gosse, 1851) | Pelagic | constant | 83.33 | Trichocerca insulana (Hauer, 1937) | Pelagic | rare | 6.67 |
| Keratella quadrata (Müller, 1786) | Pelagic | accessory | 40.00 | Trichocerca sp. | Pelagic | rare | 3.33 |
| Keratella tecta (Gosse, 1851) | Pelagic | accessory | 43.33 | Trichotria pocillum (Müller, 1776) | Littoral | rare | 10.00 |
| Keratella tropica (Apstein, 1907) | Pelagic | rare | 6.67 | ||||
| Cladocera | |||||||
| Alona guttata Sars, 1862 | Littoral | rare | 6.67 | Diaphanosoma mongolianum Ueno, 1938 | Pelagic | accessory | 43.33 |
| Biapertura affinis (Leydig, 1860) | Littoral | rare | 16.67 | Diaphanosoma macedonicum Korovchinsky and Petkovski, 2014 | Pelagic | rare | 3.33 |
| Bosmina (Bosmina) longirostris (O. F. Müller, 1776) | Pelagic | constant | 100.00 | Disparalona rostrata (Koch, 1841) | Littoral | rare | 3.33 |
| Ceriodaphnia reticulata (Jurine, 1820) | Littoral | accessory | 46.67 | Leptodora kindtii(Focke, 1844) | Pelagic | accessory | 40.00 |
| Ceriodaphnia sp. | Littoral | rare | 3.33 | Leydigia leydigi (Schödler, 1863) | Littoral | accessory | 36.67 |
| Chydorus sphaericus (O. F. Müller, 1776) | Littoral | constant | 53.33 | Macrothrix laticornis (Jurine, 1820) | Littoral | rare | 3.33 |
| Coronatella rectangula (Sars, 1862) | Littoral | rare | 10.00 | Moina micrura Kurz, 1875 | Pelagic | accessory | 36.67 |
| Daphnia (Daphnia) cucullata Sars, 1862 | Pelagic | constant | 100.00 | Simocephalus expinosus (De Geer, 1778) | Littoral | rare | 3.33 |
| Daphnia (Daphnia) pulicaria Forbes, 1983 | Pelagic | accessory | 43.33 | Simocephalus vetulus (O. F. Müller, 1776) | Littoral | rare | 3.33 |
| Copepoda | |||||||
| Acanthocyclops robustus group | Pelagic/Littoral | constant | 76.67 | Macrocyclops albidus albidus (Jurine, 1820) | Littoral | rare | 10.00 |
| Cyclops abyssorum group | Littoral | accessory | 33.33 | Mesocyclops leuckarti leuckarti (Claus, 1857) | Pelagic | constant | 73.33 |
| Cyclops vicinus vicinus Uljanin, 1875 | Pelagic | constant | 73.33 | Megacyclops viridis viridis (Jurine, 1820) | Littoral | accessory | 26.67 |
| Eucyclops serrulatus (Fischer, 1851) | Littoral | rare | 3.33 | Harpacticoida | Littoral | rare | 3.33 |
| Eudiaptomus padanus etruscus (Losito, 1901) | Pelagic | constant | 100.00 | ||||
| Ostracoda | Littoral | rare | 3.33 |
| Turnover | Nestedness | |
|---|---|---|
| Impoundment—1st year | 0.84 | 0.16 |
| Impoundment—2nd year warm | 0.93 | 0.07 |
| Impoundment—2nd year cold | 0.75 | 0.25 |
| 1st year—2nd year cold | 0.56 | 0.44 |
| 1st year—2nd year warm | 0.76 | 0.25 |
| 2nd year warm—2nd year cold | 0.65 | 0.35 |
| Parameters | Units | Mean | Stdev | Min | Max |
|---|---|---|---|---|---|
| Maximum depth | m | 105.41 | 17.57 | 47 | 125 |
| Secchi depth | m | 2.43 | 0.94 | 1.1 | 4.2 |
| T | °C | 16.43 | 6.33 | 7.5 | 25.3 |
| pH | 8.47 | 0.21 | 7.94 | 8.77 | |
| Electrical conductivity | μS/cm | 409 | 68 | 349 | 658 |
| NH4+ | (mg N-NH4/L) | 0.020 | 0.031 | <0.005 | 0.141 |
| NO2− | (mg N-NO2/L) | 0.005 | 0.005 | <0.002 | 0.015 |
| NO3− | (mg N-NO3/L) | 0.542 | 0.400 | 0.120 | 1.503 |
| TN | (mg N/L) | 1.04 | 0.96 | 0.33 | 3.67 |
| SRP | (mg P/L) | 0.004 | 0.004 | <0.002 | 0.019 |
| TP | (mg P/L) | 0.024 | 0.017 | <0.002 | 0.060 |
| TOC | (mg/L) | 2.91 | 1.18 | 1.91 | 7.91 |
| Alkalinity (pH 8.3) | (mg CaCO3/L) | 7.0 | 4.0 | 0 | 15 |
| Alkalinity (pH 4.5) | (mg CaCO3/L) | 181 | 36 | 106 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamou, G.; Katsiapi, M.; Demertzioglou, M.; Voutsa, D.; Kozari, A.; Pantelaki, I.; Moustaka-Gouni, M.; Michaloudi, E. Pelagial Zooplankton Community in a Newly Established Reservoir during and after the Impoundment of a Hydropower Dam. Diversity 2023, 15, 257. https://0-doi-org.brum.beds.ac.uk/10.3390/d15020257
Stamou G, Katsiapi M, Demertzioglou M, Voutsa D, Kozari A, Pantelaki I, Moustaka-Gouni M, Michaloudi E. Pelagial Zooplankton Community in a Newly Established Reservoir during and after the Impoundment of a Hydropower Dam. Diversity. 2023; 15(2):257. https://0-doi-org.brum.beds.ac.uk/10.3390/d15020257
Chicago/Turabian StyleStamou, Georgia, Matina Katsiapi, Maria Demertzioglou, Dimitra Voutsa, Argyri Kozari, Ioanna Pantelaki, Maria Moustaka-Gouni, and Evangelia Michaloudi. 2023. "Pelagial Zooplankton Community in a Newly Established Reservoir during and after the Impoundment of a Hydropower Dam" Diversity 15, no. 2: 257. https://0-doi-org.brum.beds.ac.uk/10.3390/d15020257