Integration of P-CuO Thin Sputtered Layers onto Microsensor Platforms for Gas Sensing
Abstract
:1. Introduction
2. Experimental
3. Preparation of Microheaters
4. Integration of P-Type CuO Layer by Photolithography Process
4.1. Structural Characterizations of CuO Layer
4.2. Description of the Integration Process
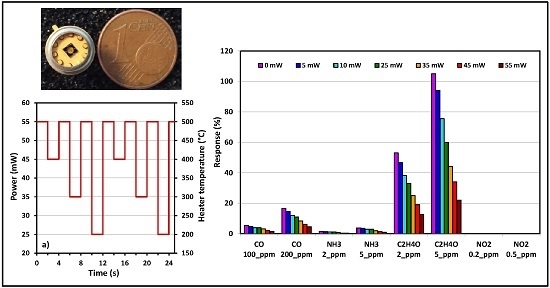
5. Sensing Tests
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demarne, V.; Grisel, A. An integrated low-power thin-film CO gas sensor on silicon. Sens. Actuators 1988, 13, 301–313. [Google Scholar] [CrossRef]
- Courbat, J.; Canonica, M.; Teyssieux, D.; Briand, D.; de Rooijet, N.F. Design and fabrication of micro-hotplates made on a polyimide foil: Electrothermal simulation and characterization to achieve power consumption in the low mW range. J. Micromech. Microeng. 2010, 21, 015014. [Google Scholar] [CrossRef]
- Briand, D.; Courbat, J. Chapter 6: Micromachined semiconductor gas sensors. In Semiconductor Gas Sensors; Jaaniso, R., Tan, O.K., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 220–260. ISBN 9780857092366. [Google Scholar]
- Sears, W.M.; Colbow, K.; Consadori, F. General characteristics of thermally cycled tin oxide gas Sensors. Semicond. Sci. Technol. 1989, 4, 351–359. [Google Scholar]
- Ratton, L.; Kunt, T.; McAvoy, T.; Fuja, T.; Cavicchi, R.; Semancik, S. A comparative study of signal processing techniques for clustering microsensor data (a first step towards an artificial nose). Sens. Actuators B Chem. 1997, 41, 105–120. [Google Scholar] [CrossRef]
- Rogers, P.H.; Semancik, S. Development of optimization procedures for application-specific chemical sensing. Sens. Actuators B Chem. 2012, 163, 8–19. [Google Scholar] [CrossRef]
- Llobet, E.; Brezmes, J.; Ionescu, R.; Vilanova, X.; Al-Khalifa, S.; Gardner, J.W.; Bârsan, N.; Correig, X. Wavelet transform and fuzzy ARTMAP-based pattern recognition for fast gas identification using a micro-hotplate gas sensor. Sens. Actuators B Chem. 2002, 83, 238–244. [Google Scholar] [CrossRef]
- Parret, F.; Ménini, Ph.; Martinez, A.; Soulantica, K.; Maisonnat, A.; Chaudret, B. Improvement of Micromachined SnO2 Gas Sensors Selectivity by Optimised Dynamic Temperature Operating Mode. Sens. Actuators B Chem. 2006, 118, 276–282. [Google Scholar] [CrossRef]
- Faglia, G.; Comini, E.; Cristalli, A.; Sberveglieri, G.; Dori, L. Very low power consumption micromachined CO sensors. Sens. Actuators B Chem. 1999, 55, 140–146. [Google Scholar] [CrossRef]
- Fong, C.-F.; Dai, C.-L.; Wu, C.-C. Fabrication and Characterization of a Micro Methanol Sensor Using the CMOS-MEMS Technique. Sensors 2015, 15, 27047–27059. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.J.; Hockey, B.; Montgomery, C.B.; Semancik, S. Porous tin oxide nanostructured microspheres for sensor applications. Langmuir 2005, 21, 7937–7944. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-Z.; Dai, C.-L.; Yang, M.-Z. Micro Ethanol Sensors with a Heater Fabricated Using the Commercial 0.18 μm CMOS Process. Sensors 2013, 13, 12760–12770. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Z.; Dai, C.-L. Ethanol Microsensors with a Readout Circuit Manufactured Using the CMOS-MEMS Technique. Sensors 2015, 15, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Chandra, S. An innovative gas sensor incorporating ZnO–CuO nanoflakes in planar MEMS technology. Sens. Actuators B Chem. 2016, 229, 414–424. [Google Scholar] [CrossRef]
- Stankova, M.; Ivanov, P.; Llobet, E.; Brezmes, J.; Vilanova, X.; Gràcia, I.; Cané, C.; Hubalek, J.; Malysz, K.; Correig, X. Sputtered and screen-printed metal oxide-based integrated micro-sensor arrays for the quantitative analysis of gas mixtures. Sens. Actuators B Chem. 2004, 103, 23–30. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Chiang, C.-M.; Wang, Y.-H.; Ma, R.-H. A self-heating gas sensor with integrated NiO thin-film for formaldehyde detection. Sens. Actuators B Chem. 2007, 122, 503–510. [Google Scholar] [CrossRef]
- Stankova, M.; Vilanova, X.; Calderer, J.; Llobet, E.; Ivanov, P.; Gràcia, I.; Cané, C.; Correig, X. Detection of SO2 and H2S in CO2 stream by means of WO3-based micro-hotplate sensors. Sens. Actuators B Chem. 2004, 102, 219–225. [Google Scholar] [CrossRef]
- Tang, Z.; Fung, S.K.H.; Wong, D.T.W.; Chan, P.C.H.; Sin, J.K.O.; Cheung, P.W. An integrated gas sensor based on tin oxide thin-film and improved micro-hotplate. Sens. Actuators B Chem. 1998, 46, 174–179. [Google Scholar] [CrossRef]
- Sheng, L.Y.; Tang, Z.; Wu, J.; Chan, P.C.H.; Sin, J.K.O. A low-power CMOS compatible integrated gas sensor using maskless tin oxide sputtering. Sens. Actuators B Chem. 1998, 49, 81–87. [Google Scholar] [CrossRef]
- Takács, M.; Dücső, C.; Pap, A.E. Fine-tuning of gas sensitivity by modification of nano-crystalline WO3 layer morphology. Sens. Actuators B Chem. 2015, 221, 281–289. [Google Scholar] [CrossRef]
- Zappa, D.; Briand, D.; Comini, E.; Courbat, J.; de Rooij, N.F.; Sberveglieri, G. Zinc Oxide Nanowires Deposited on Polymeric Hotplates for Low-power Gas Sensors. Procedia Eng. 2012, 47, 1137–1140. [Google Scholar] [CrossRef]
- Yang, M.Z.; Dai, C.L.; Shih, P.J.; Chen, Y.C. Cobalt oxide nanosheet humidity sensor integrated with circuit on chip. Microelectron. Eng. 2011, 88, 1742–1744. [Google Scholar] [CrossRef]
- Dai, C.L.; Chen, Y.C.; Wu, C.C.; Kuo, C.F. Cobalt oxide nanosheet and CNT micro carbon monoxide sensor integrated with readout circuit on chip. Sensors 2010, 10, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, J. Methanol sensing micro-gas sensors of SnO2-ZnO nanofibers on Si/SiO2/Ti/Pt substrate via stepwise-heating electrospinning. J. Material. Sci. 2015, 50, 4209–4220. [Google Scholar] [CrossRef]
- Kuhne, S.; Graf, M.; Tricoli, A.; Mayer, F.; Pratsinis, S.E.; Hierlemann, A. Wafer-level flame-spray-pyrolysis deposition of gas-sensitive layers on microsensors. J. Micromech. Microeng. 2008, 18, 035040. [Google Scholar] [CrossRef]
- Wan, Q.; Li, Q.H.; Chen, Y.J.; Wang, T.H.; He, X.L.; Li, J.P.; Lin, C.L. Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors. Appl. Phys. Lett. 2004, 84, 3654–3656. [Google Scholar] [CrossRef]
- Nguyen, H.; Quy, C.T.; Hoa, N.D.; Lam, N.T.; Duy, N.V.; Quang, V.V.; Hieu, N.V. Controllable growth of ZnO nanowire grown on discrete islands of Au catalyst for realization of planar type micro gas sensors. Sens. Actuators B Chem. 2014, 193, 888–894. [Google Scholar] [CrossRef]
- Pandya, H.J.; Chandra, S.; Vyas, A.L. Integration of ZnO nanostructures with MEMS for ethanol sensor. Sens. Actuators B Chem. 2011, 161, 923–928. [Google Scholar] [CrossRef]
- Peng, K.Q.; Wang, X.; Lee, S.T. Gas sensing properties of single crystalline porous silicon nanowires. Appl. Phys. Lett. 2009, 95, 243112. [Google Scholar] [CrossRef]
- Dong, K.Y.; Choi, J.K.; Hwang, I.S.; Lee, J.W.; Kang, B.H.; Ham, D.J.; Lee, J.H.; Ju, B.K. Enhanced H2S sensing characteristics of Pt doped SnO2 nanofibers sensors with micro heater. Sens. Actuators B Chem. 2011, 157, 154–161. [Google Scholar] [CrossRef]
- Oudrhiri-Hassani, F.; Presmanes, L.; Barnabé, A.; Tailhades, P.H. Microstructure, porosity and roughness of RF sputtered oxide thin films: Characterization and modelization. Appl. Surf. Sci. 2008, 254, 5796–5802. [Google Scholar] [CrossRef]
- Sandu, I.; Presmanes, L.; Alphonse, P.; Tailhades, P. Nanostructured cobalt manganese ferrite thin films for gas sensor application. Thin Sol. Films 2006, 495, 130–133. [Google Scholar] [CrossRef]
- Chapelle, A.; El Younsi, I.; Vitale, S.; Thimont, Y.; Nelis, T.; Presmanes, L.; Barnabé, A.; Tailhades, P.H. Improved semiconducting CuO/CuFe2O4 nanostructured thin films for CO2 gas sensing. Sens. Actuators B Chem. 2014, 204, 407–413. [Google Scholar] [CrossRef]
- Chapelle, A.; Yaacob, M.; Pasquet, I.; Presmanes, L.; Barnabe, A.; Tailhades, P.H.; Du Plessis, J.; Kalantar, K. Structural and gas-sensing properties of CuO–CuxFe3−xO4 nanostructured thin films. Sens. Actuators B Chem. 2011, 153, 117–124. [Google Scholar] [CrossRef]
- Presmanes, L.; Chapelle, A.; Oudrhiri-Hassani, F.; Barnabe, A.; Tailhades, P.H. Synthesis and CO Gas-Sensing Properties of CuO and Spinel Ferrite Nanocomposite Thin Films. Sens. Lett. 2013, 9, 587–590. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Choi, G.M. Nonstoichiometry and electrical conduction of CuO. J. Phys. Chem. Solids 1996, 57, 81–84. [Google Scholar] [CrossRef]
- Koffyberg, F.P.; Benko, F.A. A photoelectrochemical determination of the position of the conduction and valence band edges of p-type CuO. J. Appl. Phys. 1982, 53, 1173–1177. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, H.; Xiao, Y.; Liu, Y.; Yuan, A.; Shen, X. Facile fabrication and enhanced sensing properties of hierarchically porous CuO architectures. ACS Appl. Mater. Interfaces 2012, 4, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, K.; Hartman, L.; Zhou, W. H2S detection by vertically aligned CuO nanowire array sensors. J. Phys. Chem. C 2008, 112, 16017–16021. [Google Scholar] [CrossRef]
- Steinhauer, S.; Brunet, E.; Maier, T.; Mutinati, G.C.; Köck, A. Suspended CuO nanowires for ppb level H2S sensing in dry and humid atmosphere. Sens. Actuators B Chem. 2013, 186, 550–556. [Google Scholar] [CrossRef]
- Ramgir, N.S.; Kailasa Ganapathi, S.; Kaur, M.; Datta, N.; Muthe, K.P.; Aswal, D.K.; Gupta, S.K.; Yakhmi, J.V. Sub-ppm H2S sensing at room temperature using CuO thin films. Sens. Actuators B Chem. 2010, 151, 90–96. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, F.; Chen, Y.; Zhou, Y.; Li, J.; Zhu, A.; Luo, Y.; Tian, Y.; Yang, J. Hierarchically porous CuO hollow spheres fabricated via a one-pot template-free method for high-performance gas sensors. J. Phys. Chem. C 2012, 116, 11994–12000. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Lei, Y.; Gu, Z. Highly sensitive H2S sensor based on template-synthesized CuO nanowires. RSC Adv. 2012, 2, 2302–2307. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, A.; Luo, Y.; Tian, Y.; Yang, J.; Qin, Y. CuO nanosheets for sensitive and selective determination of H2S with high recovery ability. J. Phys. Chem. C 2010, 114, 19214–19219. [Google Scholar] [CrossRef]
- Yang, C.; Su, X.; Xiao, F.; Jian, J.; Wang, J. Gas sensing properties of CuO nanorods synthesized by a microwave-assisted hydrothermal method. Sens. Actuators B Chem. 2011, 158, 299–303. [Google Scholar] [CrossRef]
- Steinhauer, S.; Brunet, E.; Maier, T.; Mutinati, G.C.; Köck, A.; Freudenberg, O.; Gspan, C.; Grogger, W.; Neuhold, A.; Resel, R. Gas sensing properties of novel CuO nanowire devices. Sens. Actuators B Chem. 2013, 187, 50–57. [Google Scholar] [CrossRef]
- Aslani, A.; Oroojpour, V. CO gas sensing of CuO nanostructures synthesized by an assisted solvothermal wet chemical route. Physica B Condens Matter 2011, 406, 144–149. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Z.; Yan, B.; Zheng, Z.; Bao, Q.L.; Wu, T.; Li, C.M.; Shen, Z.X.; Zhang, J.X.; Gong, H.; et al. Multifunctional CuO nanowire devices: P-type field effect transistors and CO gas sensors. Nanotechnology 2009, 20, 085203. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Hwang, I.S.; Kim, S.J.; Lee, C.Y.; Lee, J.H. CuO nanowire gas sensors for air quality control in automotive cabin. Sens. Actuators B 2008, 135, 298–303. [Google Scholar] [CrossRef]
- Kim, K.M.; Jeong, H.M.; Kim, H.R.; Choi, K.I.; Kim, H.J.; Lee, J.H. Selective detection of NO2 using Cr-doped CuO nanorods. Sensors 2012, 12, 8013–8025. [Google Scholar] [CrossRef] [PubMed]
- Raksa, P.; Gardchareon, A.; Chairuangsri, T.; Mangkorntong, P.; Mangkorntong, N.; Choopun, S. Ethanol sensing properties of CuO nanowires prepared by an oxidation reaction. Ceram. Int. 2009, 35, 649–652. [Google Scholar] [CrossRef]
- Hsueh, H.T.; Chang, S.J.; Hung, F.Y.; Weng, W.Y.; Hsu, C.L.; Hsueh, T.J.; Lin, S.S.; Dai, B.T. Ethanol gas sensor of crabwise CuO nanowires prepared on glass substrate. J. Electrochem. Soc. 2011, 158, J106–J109. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Ahmad, M.Z.; Rani, R.A.; Ou, J.Z.; Balendhran, S.; Zhuiykov, S.; Latham, K.; Wlodarski, W.; Kalantar-zadeh, K. Nanostructured copper oxides as ethanol vapour sensors. Sens. Actuators B Chem. 2013, 185, 620–627. [Google Scholar] [CrossRef]
- Mashock, M.; Yu, K.; Cui, S.; Mao, S.; Lu, G.; Chen, J. Modulating gas sensing properties of CuO nanowires through creation of discrete nanosized p–n junctions on their surfaces. ACS Appl. Mater. Interfaces 2012, 4, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Walden, P.; Kneer, J.; Knobelspies, S.; Kronast, W.; Mescheder, U.; Palzer, S. Micromachined Hotplate Platform for the Investigation of Ink-Jet Printed, Functionalized Metal Oxide Nanoparticles. J. Microelectromech. Syst. 2015, 24, 1384–1390. [Google Scholar] [CrossRef]
- Kneer, J.; Knobelspies, S.; Bierer, B.; Wöllenstein, J.; Palzer, S. New method to selectively determine hydrogen sulfide concentrations using CuO layers. Sens. Actuators B Chem. 2016, 222, 625–631. [Google Scholar] [CrossRef]
- Presmanes, L.; Thimont, Y.; Chapelle, A.; Blanc, F.; Talhi, C.; Bonningue, C.; Barnabé, A.; Menini, P.H.; Tailhades, P.H. Highly Sensitive Sputtered ZnO:Ga Thin Films Integrated by a Simple Stencil Mask Process on Microsensor Platforms for Sub-ppm Acetaldehyde Detection. Sensors 2017, 17, 1055. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.A.; Le Trong, H.; Presmanes, L.; Barnabé, A.; Bonningue, C.; Tailhades, P.H. Thin films of Co1.7Fe1.3O4 prepared by radio-frequency sputtering—First step towards their spinodal decomposition. CrystEngComm 2014, 16, 3359–3365. [Google Scholar] [CrossRef]
- Le Trong, H.; Bui, T.M.A.; Presmanes, L.; Barnabé, A.; Pasquet, I.; Bonningue, C.; Tailhades, P.H. Preparation of iron cobaltite thin films by RF magnetron sputtering. Thin Solid Films 2015, 589, 292–297. [Google Scholar] [CrossRef]
- Shang, C.; Thimont, Y.; Barnabe, A.; Presmanes, L.; Pasquet, I.; Tailhades, P.H. Detailed microstructure analysis of as-deposited and etched porous ZnO films. Appl. Surf. Sci. 2015, 344, 242–248. [Google Scholar] [CrossRef]
- Menini, P. Habilitation à Diriger les Recherches; Université de Toulouse: Toulouse, French, 2011; (Figure 51 page 97, and Figure 54 page 101); Available online: https://tel.archives-ouvertes.fr/tel-00697471/document (accessed on 12 May 2017).
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational properties of CuO and Cu4O3 from first-principles calculations, and Raman and infrared spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.H. Binary copper oxide semiconductors: From materials towards devices. Phys. Status Solidi 2012, 249, 1487–1509. [Google Scholar] [CrossRef]
- ANSES Website. Available online: https://www.anses.fr/en (accessed on 16 May 2017).
- World Health Organization (WHO) website. WHO Guidelines for Indoor Air Quality: Selected Pollutants. 2010. Available online: http://www.who.int/en/ (accessed on 16 May 2017).
- Kim, K.-M.; Jeong, H.-M.; Kim, H.-R.; Choi, K.-I.; Kim, H.-J.; Lee, J.-H. Selective Detection of NO2 Using Cr-Doped CuO Nanorods. Sensors 2012, 12, 8013–8025. [Google Scholar] [CrossRef] [PubMed]
- Cordi, E.M.; O’Neill, P.J.; Falconer, J.L. Transient oxidation of volatile organic compounds on a CuO/Al2O3 catalyst. Appl. Catal. B Environ. 1997, 14, 23–36. [Google Scholar] [CrossRef]
- Kunt, T.A.; McAvoy, T.J.; Cavicchi, R.E.; Semancik, S. Optimization of temperature programmed sensing for gas identification using micro-hotplate sensors. Sens. Actuators B Chem. 1998, 53, 24–43. [Google Scholar] [CrossRef]
- Tropis, C.; Menini, P.; Martinez, A.; Yoboue, N.; Franc, B.; Blanc, F.; Fadel, P.; Lagrange, D.; Fau, P.; Maisonnat, A. Characterization of dynamic measurement with nanoparticular SnO2 gas sensors. In Proceedings of the 20th MicroMechanics Europe Workshop (MME 2009), Toulouse, France, 20–22 September 2009. [Google Scholar]
- Ionescu, R.; Llobet, E.; Al-Khalifa, S.; Gardner, J.W.; Vilanova, X.; Brezmes, J.; Correig, X. Response model for thermally modulated tin oxide-based microhotplate gas sensors. Sens. Actuators B Chem. 2003, 95, 203–211. [Google Scholar] [CrossRef]
- Ducéré, J.-M.; Hemeryck, A.; Estève, A.; Rouhani, M.D.; Landa, G.; Ménini, P.; Tropis, C.; Maisonnat, A.; Fau, P.; Chaudret, B. A computational chemist approach to gas sensors: Modeling the response of SnO2 to CO, O2, and H2O Gases. J. Comput. Chem. 2012, 33, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Ruhland, B.; Becker, T.H.; Müller, G. Gas-kinetic interactions of nitrous oxides with SnO2 surfaces. Sens. Actuators B Chem. 1998, 50, 85–94. [Google Scholar] [CrossRef]
- Kneer, J.; Wöllenstein, J.; Palzer, S. Manipulating the gas–surface interaction between copper(II) oxide and mono-nitrogen oxides using temperature. Sens. Actuators B Chem. 2016, 229, 57–62. [Google Scholar] [CrossRef]














| Target material | CuO |
| Magnetron | Yes |
| Substrates | Fused silica and micro-hotplate |
| Power | 50 W |
| Argon pressure | 0.5 Pa |
| Target to substrate distance | 7 cm |
| Deposition rate | 6.1 nm/min |
| Power (mW) | Temperature (°C) |
|---|---|
| 55 | 500.7 |
| 45 | 402.7 |
| 35 | 304.8 |
| 30 | 255.8 |
| 25 | 206.9 |
| Gas | Concentration (ppm) | |
|---|---|---|
| CO | 100 | 200 |
| NH3 | 2 | 5 |
| C2H4O | 2 | 5 |
| NO2 | 0.2 | 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presmanes, L.; Thimont, Y.; El Younsi, I.; Chapelle, A.; Blanc, F.; Talhi, C.; Bonningue, C.; Barnabé, A.; Menini, P.; Tailhades, P. Integration of P-CuO Thin Sputtered Layers onto Microsensor Platforms for Gas Sensing. Sensors 2017, 17, 1409. https://0-doi-org.brum.beds.ac.uk/10.3390/s17061409
Presmanes L, Thimont Y, El Younsi I, Chapelle A, Blanc F, Talhi C, Bonningue C, Barnabé A, Menini P, Tailhades P. Integration of P-CuO Thin Sputtered Layers onto Microsensor Platforms for Gas Sensing. Sensors. 2017; 17(6):1409. https://0-doi-org.brum.beds.ac.uk/10.3390/s17061409
Chicago/Turabian StylePresmanes, Lionel, Yohann Thimont, Imane El Younsi, Audrey Chapelle, Frédéric Blanc, Chabane Talhi, Corine Bonningue, Antoine Barnabé, Philippe Menini, and Philippe Tailhades. 2017. "Integration of P-CuO Thin Sputtered Layers onto Microsensor Platforms for Gas Sensing" Sensors 17, no. 6: 1409. https://0-doi-org.brum.beds.ac.uk/10.3390/s17061409