Study of Room Temperature Ionic Liquids as Gas Sensing Materials in Quartz Crystal Microbalances
Abstract
:1. Introduction
2. Materials and Methods
3. Results
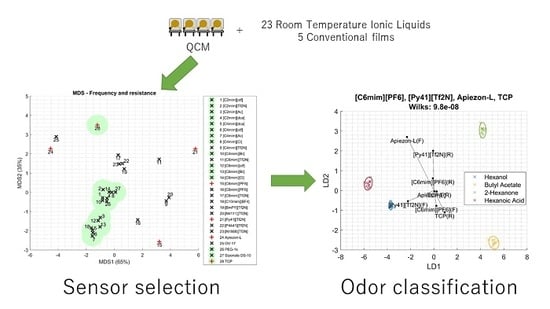
3.1. Measurements
3.2. RTIL Sensors
3.3. Conventional Films
3.4. All Sensors Together
3.5. Validation of Sensor Array with Two Aromas
4. Discussion
5. Concussions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Jasinski, G.; Wozniak, L.; Kalinowski, P.; Jasinski, P. Evaluation of the electronic nose used for monitoring environmental pollution. In Proceedings of the 2018 XV International Scientific Conference on Optoelectronic and Electronic Sensors (COE), Warsaw, Poland, 17–20 June 2018; pp. 1–4. [Google Scholar]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Farraia, M.V.; Cavaleiro Rufo, J.; Paciência, I.; Mendes, F.; Delgado, L.; Moreira, A. The electronic nose technology in clinical diagnosis. Porto Biomed. J. 2019, 4, e42. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Luo, D. A method of olfactory display: Odor characterization and reproduction. In Proceedings of the 2017 ISOCS/IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Montreal, QC, Canada, 28–31 May 2017; pp. 1–3. [Google Scholar]
- Cluster-essc. Available online: http://www.cluster-essc.eu/ (accessed on 20 July 2020).
- Wang, L.; Lou, Z.; Zhang, R.; Zhou, T.; Deng, J.; Zhang, T. Hybrid Co3O4/SnO2 core–shell nanospheres as real-time rapid-response sensors for ammonia gas. ACS Appl. Mater. Interfaces 2016, 8, 6539–6545. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, F.; Huang, D.; Di, C.; Meng, Q.; Gao, X.; Zhu, D. Specific and reproducible gas sensors utilizing gas-phase chemical reaction on organic transistors. Adv. Mater. 2014, 26, 2862–2867. [Google Scholar] [CrossRef]
- Kumar, P.; Morawska, L.; Martani, C.; Biskos, G.; Neophytou, M.; Di Sabatino, S.; Bell, M.; Norford, L.; Britter, R. The rise of low-cost sensing for managing air pollution in cities. Environ. Int. 2015, 75, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleixandre, M.; Santos, J.P.; Sayago, I.; Cabellos, J.M.; Arroyo, T.; Horrillo, M.C. A wireless and portable electronic nose to differentiate musts of different ripeness degree and grape varieties. Sensors 2015, 15, 8429–8443. [Google Scholar] [CrossRef] [Green Version]
- Längkvist, M.; Karlsson, L.; Loutfi, A. A review of unsupervised feature learning and deep learning for time-series modeling. Pattern Recognit. Lett. 2014, 42, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Bernabei, M.; Persaud, K.C.; Pantalei, S.; Zampetti, E.; Beccherelli, R. Large-scale chemical sensor array testing biological olfaction concepts. IEEE Sens. J. 2012, 12, 3174–3183. [Google Scholar] [CrossRef]
- Pineau, N.J.; Kompalla, J.F.; Güntner, A.T.; Pratsinis, S.E. Orthogonal gas sensor arrays by chemoresistive material design. Microchim. Acta 2018, 185, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A.; Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ema, K.; Yokoyama, M.; Nakamoto, T.; Moriizumi, T. Odour-sensing system using a quartz-resonator sensor array and neural-network pattern recognition. Sens. Actuators 1989, 18, 291–296. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakamoto, T.; Moriizumi, T. Classification and evaluation of sensing films for QCM odor sensors by steady-state sensor response measurement. Sens. Actuators B Chem. 2000, 69, 295–301. [Google Scholar] [CrossRef]
- Nakamura, K. Evaluation of relationship between odorant molecular structures and responses of QCM odor sensors. In Proceedings of the Technical Digest of the 17th Sensor Symposium, Kawasaki, Japan, 30–31 May 2000; pp. 307–312. [Google Scholar]
- King, W.H. Piezoelectric Sorption Detector. Anal. Chem. 1964, 36, 1735–1739. [Google Scholar] [CrossRef]
- McGinn, C.K.; Lamport, Z.A.; Kymissis, I. Review of gravimetric sensing of volatile organic compounds. ACS Sens. 2020. [Google Scholar] [CrossRef] [PubMed]
- Matatagui, D.; Fontecha, J.; Fernández, M.J.; Aleixandre, M.; Grcia, I.; Cané, C.; Horrillo, M.C. Array of Love-wave sensors based on quartz/Novolac to detect CWA simulants. Talanta 2011, 85, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.L.; Edrees, H.; Kymissis, I.; Shepard, K.L. Integrated VOC vapor sensing on FBAR-CMOS array. In Proceedings of the 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), Paris, France, 29 Janurary–2 February 2012; pp. 846–849. [Google Scholar]
- Bao, Y.; Xu, P.; Cai, S.; Yu, H.; Li, X. Detection of volatile-organic-compounds (VOCs) in solution using cantilever-based gas sensors. Talanta 2018, 182, 148–155. [Google Scholar] [CrossRef]
- Speller, N.C.; Siraj, N.; Vaughan, S.; Speller, L.N.; Warner, I.M. Assessment of QCM array schemes for mixture identification: Citrus scented odors. RSC Adv. 2016, 6, 95378–95386. [Google Scholar] [CrossRef]
- Koide, N.; Ichifuji, Y. Fragrance to vector as scent technology. In Proceedings of the 2017 IEEE International Conference on Big Data (Big Data), Boston, MA, USA, 11–14 December 2017. [Google Scholar]
- Korotcenkov, G. Materials for piezoelectric-based gas sensors. In Handbook of Gas Sensor Materials; Springer: New York, NY, USA, 2013; pp. 307–328. [Google Scholar]
- Marsh, K.; Boxall, J.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilib. 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Deenadayalu, N.; Thango, S.H.; Letcher, T.M.; Ramjugernath, D. Measurement of activity coefficients at infinite dilution using polar and non-polar solutes in the ionic liquid 1-methyl-3-octyl-imidazolium diethyleneglycolmonomethylethersulfate at T = (288.15, 298.15, and 313.15) K. J. Chem. Thermodyn. 2006, 38, 542–546. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Methods and approaches of utilizing ionic liquids as gas sensing materials. RSC Adv. 2015, 5, 58371–58392. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.; Pei, K.; Zhao, K.; Zhao, Z.K.; Li, H. Ionic liquids used as QCM coating materials for the detection of alcohols. Sens. Actuators B Chem. 2008, 134, 258–265. [Google Scholar] [CrossRef]
- Mikami, K. Green Reaction Media in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Ab Rani, M.A.; Brant, A.; Crowhurst, L.; Dolan, A.; Lui, M.; Hassan, N.H.; Hallett, J.P.; Hunt, P.A.; Niedermeyer, H.; Perez-Arlandis, J.M.; et al. Understanding the polarity of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 16831–16840. [Google Scholar] [CrossRef]
- Shinwa Chemical Industries Ltd. Packed Columns Stationary Phases. Available online: https://shinwa-cpc.co.jp/en/products/gc/packed/stationary/ (accessed on 3 March 2020).
- Zondlo, M.M. Final report on the safety assessment of sodium Dodecylbenzenesulfonatel TEA-Dodecy l benzenesu Ifonate/ Sodium Decyl benzenesulfonate. J. Am. Coll. Toxicol. 1993, 12, 279–309. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z. Für Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Aleixandre, M.; Nakazawa, K.; Nakamoto, T. Optimization of modulation methods for solenoid valves to realize an odor generation system. Sensors 2019, 19, 4009. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, T.; Minh, H.P.D. Improvement of olfactory display using solenoid valves. In Proceedings of the 2007 IEEE Virtual Reality Conference, Charlotte, NC, USA, 10–14 March 2007; pp. 179–186. [Google Scholar]
- Yamanaka, T.; Matsumoto, R.; Nakamoto, T. Study of recording apple flavor using odor recorder with five components. Sens. Actuators B Chem. 2003, 89, 112–119. [Google Scholar] [CrossRef]
- Scaringelli, F.P.; O’Keeffe, A.E.; Rosenberg, E.; Bell, J.P. Preparation of known concentrations of gases and vapors with permeation devices calibrated gravimetrically. Anal. Chem. 1970, 42, 871–876. [Google Scholar] [CrossRef]
- Uncertainty of Measurement Results from NIST. Available online: https://physics.nist.gov/cuu/Uncertainty/index.html (accessed on 30 April 2020).
- Nakamoto, T.; Moriizumi, T.; Takamichi, N.; Toyosaka, M. A Theory of a quartz crystal microbalance based upon a mason equivalent circuit. Jpn. J. Appl. Phys. 1990, 29, 963–969. [Google Scholar] [CrossRef]
- Jin, X.; Huang, Y.; Mason, A.; Zeng, X. Multichannel monolithic quartz crystal microbalance gas sensor array. Anal. Chem. 2008, 81, 595–603. [Google Scholar] [CrossRef]
- Songkhla, S.N.; Nakamoto, T. Signal processing of vector network analyzer measurement for quartz crystal microbalance with viscous damping. IEEE Sens. J. 2019, 19, 10386–10392. [Google Scholar] [CrossRef]
- Wilks, S.S. Certain Generalizations in the Analysis of Variance. Biometrika 1932, 24, 471. [Google Scholar] [CrossRef]
- Nakamoto, T.; Sasaki, S.; Fukuda, A.; Moriizumi, T. Selection method of sensing membranes in odor-sensing system. Sens. Mater. 1992, 4, 111–120. [Google Scholar]
- Bechmann, R. Frequency-temperature-angle characteristics of AT-type resonators made of natural and synthetic quartz. Proc. IRE 1956, 44, 1600–1607. [Google Scholar] [CrossRef]
- Lack, F.R.; Willard, G.W.; Fair, I.E. Some improvements in quartz crystal circuit elements. Bell Syst. Tech. J. 1934, 13, 453–463. [Google Scholar] [CrossRef]
- Sun, P.; Jiang, Y.; Xie, G.; Du, X.; Hu, J. A room temperature supramolecular-based quartz crystal microbalance (QCM) methane gas sensor. Sens. Actuators B Chem. 2009, 141, 104–108. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, X.; Li, X.; Chen, X.; Li, N. Investigation of the stability of QCM humidity sensor using graphene oxide as sensing films. Sens. Actuators B Chem. 2014, 191, 779–783. [Google Scholar] [CrossRef]
- Faustini, M.; Louis, B.; Albouy, P.A.; Kuemmel, M.; Grosso, D. Preparation of Sol-Gel films by dip-coating in extreme conditions. J. Phys. Chem. C 2010, 114, 7637–7645. [Google Scholar] [CrossRef]
- Muñoz-Aguirre, S.; Nakamoto, T.; Moriizumi, T. Study of deposition of gas sensing films on quartz crystal microbalance using an ultrasonic atomizer. Sens. Actuators B Chem. 2005, 105, 144–149. [Google Scholar] [CrossRef]





| Sensing Material | Deposition Frequency Shift | ||
|---|---|---|---|
| Identifier | CAS # | Full Name | (Hz) |
| [C2mim][otf] | 145022-44-2 | 1-Ethyl-3-methylimidazolium triflate | 1688 |
| [C2mim][Tf2N] | 174899-82-2 | 1-Ethyl-3-methylimidazolium | 1184 |
| bis(trifluoromethylsulphonyl)imide | |||
| [C2mim][Ac] | 143314-17-4 | 1-Ethyl-3-methylimidazolium acetate | 1584 |
| [C2mim][dca] | 370865-89-7 | 1-Ethyl-3-methylimidazolium dicyanamide | 1514 |
| [C4mim][dca] | 448245-52-1 | 1-butyl-3-methylimidazolium dicyanamide | 1300 |
| [C4mim][otf] | 174899-66-2 | 1-Butyl-3-methylimidazolium triflate | 1149 |
| [C4mim][Ac] | 284049-75-8 | 1-Butyl-3-methylimidazolium acetate | 967 |
| [C4mim][Cl] | 79917-90-1 | 1-butyl-3-methylimidazolium chloride | 1006 |
| [C4mim][Tf2N] | 174899-83-3 | 1-butyl-3-methylimidazolium | 1509 |
| bis(trifluoromethylsulfonyl)imide | |||
| [C4mim][Br] | 85100-77-2 | 1-butyl-3-methylimidazolium bromide | 1200 |
| [C6mim][Tf2N] | 382150-50-7 | 1-hexyl-3-methylimidazolium | 1194 |
| bis[(trifluoromethyl)sulfonyl]imide | |||
| [C6mim][otf] | 460345-16-8 | 1-hexyl-3-methylimidazolium | 1268 |
| trifluoromethanesulfonate | |||
| [C6mim][PF6] | 304680-35-1 | 1-hexyl-3-methylimidazolium hexafluorophosphate | 1042 |
| [C6mim][Br] | 85100-78-3 | 3-hexyl-1-methyl-1H-imidazolium bromide | 1119 |
| [C6mim][Cl] | 171058-17-6 | 1-hexyl-3-methylimidazolium chloride | 1300 |
| [C8mim][PF6] | 304680-36-2 | 1-octyl-3-methylimidazolium | 1438 |
| Hexafluorophosphate | |||
| [C8mim][Tf2N] | 178631-04-4 | 1-octyl-3-methylimidazolium | 1175 |
| bis[(trifluoromethyl)sulfonyl]imide | |||
| [C10mim][BF4] | 244193-56-4 | 1-Decyl-3-methylimidazolium | 1055 |
| tetrafluoroborate | |||
| [BmPY][Tf2N] | 623580-02-9 | 1-Butyl-1-methylpiperidinium | 1182 |
| bis(trifluoromethylsulphonyl)imide | |||
| [N4111][Tf2N] | 324575-10-2 | Butyltrimethylammonium | 1561 |
| bis(trifluoromethanesulfonyl)imide | |||
| [Py41][Tf2N] | 223437-11-4 | 1-Butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide | 1163 |
| [P4441][Tf2N] | 324575-10-2 | Tributylmethylphosphonium | 1293 |
| bis(trifluoromethanesulfonyl)imide | |||
| [N1888][Tf2N] | 258273-75-5 | Methyltrioctylammonium | 1156 |
| bis(trifluoromethylsulphonyl)imide | |||
| TCP | 1330-78-5 | Tricresyl phosphate | 1352 |
| APIEZON-L | 8009-03-08 | Petrolatum (Vaseline) | 1339 |
| PEG 1k | 25322-68-3 | Poly(ethylene glycol) 1,000 | 1169 |
| OV-17 | 63148-58-3 | Methyl Phenyl Silicone Oil | 1179 |
| SIPONATE DS-10 | 25155-30-0 | Sodium dodecylbenzenesulfonate | 1230 |
| Gas | Hexanol | Butyl Acetate | 2-Hexanone | Hexanoic Acid |
|---|---|---|---|---|
| Concentration (ppmv) | 62.9 | 758.7 | 753.8 | 9.0 |
| Uncertainty (%) | 25.0 | 5.3 | 5.2 | 148.7 |
| RTIL | Conventional Films | All Sensors | ||||
|---|---|---|---|---|---|---|
| Sensors | Wilks Λ | Sensors | Wilks Λ | Sensors | Wilks Λ | |
| Frequency Data (4 features) | [Py41][Tf2N](F) | 3.5 × 10−5 | OV-17(F) | 1.6 × 10−4 | [Py41][Tf2N](F) | 1.3 × 10−6 |
| [C4mim][Cl](F) | Siponate DS-10(F) | [C4mim][Cl](F) | ||||
| [C4mim][otf](F) | TCP(F) | [C4mim][otf](F) | ||||
| [C4mim][Tf2N](F) | Apiezon-L(F) | OV-17(F) | ||||
| Resistance Data (4 features) | [Py41][Tf2N](R) | 1.2 × 10−4 | OV-17(R) | 4.2 × 10−3 | [Py41][Tf2N](R) | 6.8 × 10−5 |
| [C4mim][dca](R) | Siponate DS-10(R) | [C6mim][Br](R) | ||||
| [C6mim][Br](R) | TCP(R) | [C6mim][Tf2N](R) | ||||
| [C6mim][Tf2N](R) | PEG-1k(R) | TCP(R) | ||||
| Frequency and Resistance Data (8 features) | [Py41][Tf2N](F&R) | 4.6 × 10−6 | OV-17(F&R) | 9.2 × 10−6 | [Py41][Tf2N](F&R) | 9.8 × 10−8 |
| [P4441][Tf2N](F&R) | Siponate DS-10(F&R) | [C6mim][PF6](F&R) | ||||
| [C6mim][otf](F&R) | TCP(F&R) | TCP(F&R) | ||||
| [C10mim][BF4](F&R) | PEG-1k(F&R) | Apiezon-L(F&R) | ||||
| Frequency (8 features) | [C4mim][otf](F) | 5.6 × 10−6 | [Py41][Tf2N](F) | 1.7× 10−7 | ||
| [Py41][Tf2N](F) | OV-17(F) | |||||
| [C4mim][Cl](F) | [C10mim][BF4](F) | |||||
| [C4mim][Tf2N](F) | [C4mim][otf](F) | |||||
| [C6mim][Br](F) | Apiezon-L(F) | |||||
| [C4mim][Br](F) | [C4mim][Cl](F) | |||||
| [C6mim][Tf2N](F) | [C4mim][Tf2N](F) | |||||
| [N4111][Tf2N](F) | [C6mim][otf](F) | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleixandre, M.; Nakamoto, T. Study of Room Temperature Ionic Liquids as Gas Sensing Materials in Quartz Crystal Microbalances. Sensors 2020, 20, 4026. https://0-doi-org.brum.beds.ac.uk/10.3390/s20144026
Aleixandre M, Nakamoto T. Study of Room Temperature Ionic Liquids as Gas Sensing Materials in Quartz Crystal Microbalances. Sensors. 2020; 20(14):4026. https://0-doi-org.brum.beds.ac.uk/10.3390/s20144026
Chicago/Turabian StyleAleixandre, Manuel, and Takamichi Nakamoto. 2020. "Study of Room Temperature Ionic Liquids as Gas Sensing Materials in Quartz Crystal Microbalances" Sensors 20, no. 14: 4026. https://0-doi-org.brum.beds.ac.uk/10.3390/s20144026