Can Satellite Remote Sensing Assist in the Characterization of Yeasts Related to Biogeographical Origin?
Abstract
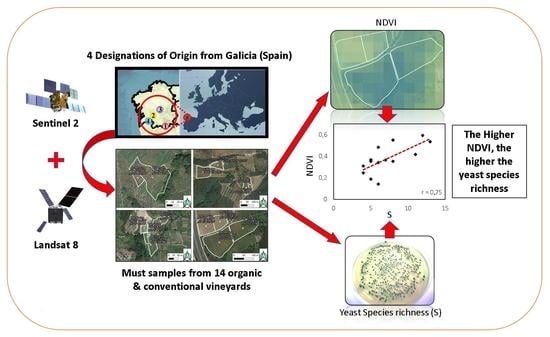
:1. Introduction
2. Materials and Methods
2.1. Experimental Trial and Yeast Identification
2.2. Sentinel-2 and Landsat-8 Multispectral Images
2.3. Data Analysis
3. Results
3.1. Previous Exploratory Analysis
3.2. In-Depth Analysis of the Microbial Terroir
4. Discussion
- In the previous paper, we used data from three years to validate the method (as an experiment to establish a link) by focusing on the terroir and year factors, while in the present document, we focus on the yeast terroir associated with vegetation for each farming system.
- In the current study, we used more complete one-year data, including other biodiversity data, such as the frequency of each yeast species, and not just the number of species.
- In the previous work, we used a satellite with a lower resolution (Landsat-8), and it was hypothesised that a higher resolution could improve detection. Therefore, in the current work, we also use Sentinel 2 (to compare them and observe the influence of resolution, including close non-identical dates, which extends the validation of the method).
- The cultivation system was not explored in depth in the previous article; therefore, it was established as an important factor of study in the present paper. Castrillo et al. [18] found that diversity was lower in grapes than in musts and, in turn, was generally lower in conventional than in organic management, samples of grapes (microbiota present on the surface of the berries), as well as musts for both farming systems.
- Furthermore, in the present work, we performed linear regressions between NDVI calculated from Landsat-8 and Sentinel-2 images and isolated yeast strains (Figure 8 and supplementary data) to assess the relationship between satellite imagery and yeasts both in number and frequency.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandre, H. Wine Yeast Terroir: Separating the Wheat from the Chaff—For an Open Debate. Microorganisms 2020, 8, 787. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate Change and Global Wine Quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Robinson, J. The Oxford Companion to Wine; Robinson, J., Harding, J., Eds.; Oxford University Press (OUP): Oxford, UK, 2006. [Google Scholar]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Vélez, S.; Barajas, E.; Rubio, J.A.; Poblete-Echeverría, C.; Olmedo, G.F. Remote Sensing: In the Digital Viticulture Era. In Vitis: Biology and Species; Jordão, A.M.S.T., Botelho, R.V., Eds.; Nova Publishers: Hauppauge, NY, USA, 2020; pp. 249–270. ISBN 978-1-53618-308-5. [Google Scholar]
- Matese, A.; Toscano, P.; Di Gennaro, S.F.; Genesio, L.; Vaccari, F.P.; Primicerio, J.; Belli, C.; Zaldei, A.; Bianconi, R.; Gioli, B. Intercomparison of UAV, Aircraft and Satellite Remote Sensing Platforms for Precision Viticulture. Remote Sens. 2015, 7, 2971–2990. [Google Scholar] [CrossRef]
- Markham, B.; Storey, J.; Morfitt, R. Landsat-8 Sensor Characterization and Calibration. Remote Sens. 2015, 7, 2279–2282. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS proceeding. In Proceedings of the Third Earth Reserves Technology Satellite Symposium, Greenbelt: NASA SP-351; NASA: Washington, DC, USA, 1974; Volume 30103017, p. 317. [Google Scholar]
- Vélez, S.; Rubio, J.A.; Andrés, M.I.; Barajas, E. Agronomic classification between vineyards (‘Verdejo’) using NDVI and Sentinel-2 and evaluation of their wines. Vitis—J. Grapevine Res. 2019, 58, 33–38. [Google Scholar] [CrossRef]
- Matese, A.; Di Gennaro, S.F. Beyond the traditional NDVI index as a key factor to mainstream the use of UAV in precision viticulture. Sci. Rep. 2021, 11, 2721. [Google Scholar] [CrossRef]
- Anastasiou, E.; Balafoutis, A.; Darra, N.; Psiroukis, V.; Biniari, A.; Xanthopoulos, G.; Fountas, S. Satellite and Proximal Sensing to Estimate the Yield and Quality of Table Grapes. Agriculture 2018, 8, 94. [Google Scholar] [CrossRef]
- Johnson, L.F.; Bosch, D.F.; Williams, D.C.; Lobitz, B.M. Remote sensing of vineyard management zones: Implications for wine quality. Appl. Eng. Agric. 2001, 17, 557. [Google Scholar] [CrossRef]
- Vélez, S.; Rançon, F.; Barajas, E.; Brunel, G.; Rubio, J.A.; Tisseyre, B. Potential of functional analysis applied to Sentinel-2 time-series to assess relevant agronomic parameters at the within-field level in viticulture. Comput. Electron. Agric. 2022, 194, 106726. [Google Scholar] [CrossRef]
- Meyers, J.M.; Dokoozlian, N.; Ryan, C.; Bioni, C.; Vanden Heuvel, J.E. A New, Satellite NDVI-Based Sampling Protocol for Grape Maturation Monitoring. Remote Sens. 2020, 12, 1159. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tasting the terroir of wine yeast innovation. FEMS Yeast Res. 2020, 20, foz084. [Google Scholar] [CrossRef]
- Vélez, S.; Barajas, E.; Blanco, P.; Rubio, J.A.; Castrillo, D. Spatio-Temporal Analysis of Satellite Imagery (NDVI) to Identify Terroir and Vineyard Yeast Differences according to Appellation of Origin (AOP) and Biogeographic Origin. Multidiscip. Sci. J. 2021, 4, 244–256. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Oenological potential of non-Saccharomyces yeasts to mitigate effects of climate change in winemaking: Impact on aroma and sensory profiles of Treixadura wines. FEMS Yeast Res. 2019, 19, foz065. [Google Scholar] [CrossRef]
- Castrillo, D.; Rabuñal, E.; Neira, N.; Blanco, P. Yeast diversity on grapes from Galicia, NW Spain: Biogeographical patterns and the influence of the farming system. Oeno One 2019, 53, 573–587. [Google Scholar] [CrossRef]
- Blanco, P.; Castrillo, D.; Graña, M.J.; Lorenzo, M.J.; Soto, E. Evaluation of autochthonous non-saccharomyces yeasts by sequential fermentation for wine differentiation in galicia (Nw spain). Fermentation 2021, 7, 183. [Google Scholar] [CrossRef]
- Castrillo, D.; Blanco, P. Influence of vintage, geographic location and agricultural management on yeast population associated to Galician grape musts (NW Spain). OENO One 2022, in press. [Google Scholar]
- Lamb, D.W.; Weedon, M.M.; Bramley, R.G.V. Using remote sensing to predict grape phenolics and colour at harvest in a Cabernet Sauvignon vineyard: Timing observations against vine phenology and optimising image resolution. Aust. J. Grape Wine Res. 2004, 10, 46–54. [Google Scholar] [CrossRef]
- Hall, A.; Lamb, D.W.; Holzapfel, B.P.; Louis, J.P. Within-season temporal variation in correlations between vineyard canopy and winegrape composition and yield. Precis. Agric. 2010, 12, 103–117. [Google Scholar] [CrossRef]
- Mathews, A.J.; Jensen, J.L.R. Visualizing and Quantifying Vineyard Canopy LAI Using an Unmanned Aerial Vehicle (UAV) Collected High Density Structure from Motion Point Cloud. Remote Sens. 2013, 5, 2164–2183. [Google Scholar] [CrossRef]
- Ledderhof, D.; Brown, R.; Reynolds, A.; Jollineau, M. Using remote sensing to understand pinot noir vineyard variability in Ontario. Can. J. Plant Sci. 2016, 96, 89–108. [Google Scholar] [CrossRef]
- Martinez-Casasnovas, J.A.; Agelet-Fernandez, J.; Arno, J.; Ramos, M.C. Analysis of vineyard differential management zones and relation to vine development, grape maturity and quality. Span. J. Agric. Res. 2012, 10, 326–337. [Google Scholar] [CrossRef]
- Teodoro, A.; Amaral, A. A Statistical and Spatial Analysis of Portuguese Forest Fires in Summer 2016 Considering Landsat 8 and Sentinel 2A Data. Environments 2019, 6, 36. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Jin, T.; Chang, X.; Zhu, Y.; Li, Y.; Sun, J.; Li, D. Comparative Analysis of Landsat-8, Sentinel-2, and GF-1 Data for Retrieving Soil Moisture over Wheat Farmlands. Remote Sens. 2020, 12, 2708. [Google Scholar] [CrossRef]
- Qin, Q.; Xu, D.; Hou, L.; Shen, B.; Xin, X. Comparing vegetation indices from Sentinel-2 and Landsat 8 under different vegetation gradients based on a controlled grazing experiment. Ecol. Indic. 2021, 133, 108363. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The microbial ecology of wine grape berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Tristezza, M.; Grieco, F.; Spano, G.; Capozzi, V. From grape berries to wine: Population dynamics of cultivable yeasts associated to “Nero di Troia” autochthonous grape cultivar. World J. Microbiol. Biotechnol. 2016, 32, 59. [Google Scholar] [CrossRef]
- Bagheri, B.; Bauer, F.F.; Setati, M.E. The diversity and dynamics of indigenous yeast communities in grape must from vineyards employing different agronomic practices and their influence on wine fermentation. South Afr. J. Enol. Vitic. 2015, 36, 243–251. [Google Scholar] [CrossRef]
- Morata, A.; Arroyo, T.; Bañuelos, M.A.; Blanco, P.; Briones, A.; Cantoral, J.M.; Castrillo, D.; Cordero-Bueso, G.; del Fresno, J.M.; Escott, C.; et al. Wine yeast selection in the Iberian Peninsula: Saccharomyces and non-Saccharomyces as drivers of innovation in Spanish and Portuguese wine industries. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef]
- Blanco, P.; Orriols, I.; Losada, A. Survival of commercial yeasts in the winery environment and their prevalence during spontaneous fermentations. J. Ind. Microbiol. Biotechnol. 2011, 38, 235–239. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef] [Green Version]
- Gayevskiy, V.; Goddard, M.R. Geographic delineations of yeast communities and populations associated with vines and wines in New Zealand. ISME J. 2012, 6, 1281–1290. [Google Scholar] [CrossRef]
- Miura, T.; Sánchez, R.; Castañeda, L.E.; Godoy, K.; Barbosa, O. Is microbial terroir related to geographic distance between vineyards? Environ. Microbiol. Rep. 2017, 9, 742–749. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef]
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The Oenological Potential of Hanseniaspora uvarum in Simultaneous and Sequential Co-fermentation with Saccharomyces cerevisiae for Industrial Wine Production. Front. Microbiol. 2016, 7, 670. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Pollon, M.; Fracassetti, D.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018, 257, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Mančić, S.; Stamenković Stojanović, S.; Danilović, B.; Djordjević, N.; Malićanin, M.; Lazić, M.; Karabegović, I. Oenological Characterization of Native Hanseniaspora uvarum Strains. Fermentation 2022, 8, 92. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High Potential of Pichia kluyveri and Other Pichia Species in Wine Technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef]
- Vicente, J.; Ruiz, J.; Belda, I.; Benito-Vázquez, I.; Marquina, D.; Calderón, F.; Santos, A.; Benito, S. The Genus Metschnikowia in Enology. Microorganisms 2020, 8, 1038. [Google Scholar] [CrossRef]
- Ramírez, M.; López-Piñeiro, A.; Velázquez, R.; Muñoz, A.; Regodón, J.A. Analysing the vineyard soil as a natural reservoir for wine yeasts. Food Res. Int. 2020, 129, 108845. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From vineyard soil to wine fermentation: Microbiome approximations to explain the “terroir” Concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef]
- Comitini, F.; Ciani, M. Influence of fungicide treatments on the occurrence of yeast flora associated with wine grapes. Ann. Microbiol. 2008, 58, 489–493. [Google Scholar] [CrossRef]
- Drumonde-Neves, J.; Franco-Duarte, R.; Lima, T.; Schuller, D.; Pais, C. Association between grape yeast communities and the vineyard ecosystems. PLoS ONE 2017, 12, e0169883. [Google Scholar] [CrossRef]
- Griggs, R.G.; Steenwerth, K.L.; Mills, D.A.; Cantu, D.; Bokulich, N.A. Sources and Assembly of Microbial Communities in Vineyards as a Functional Component of Winegrowing. Front. Microbiol. 2021, 12, 836. [Google Scholar] [CrossRef]
- Setati, M.E.; Jacobson, D.; Andong, U.C.; Bauer, F. The Vineyard Yeast Microbiome, a Mixed Model Microbial Map. PLoS ONE 2012, 7, e0052609. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the Vineyard to the Winery: How Microbial Ecology Drives Regional Distinctiveness of Wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef]
- Bokulich, N.; Collins, T.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. MBio 2016, 7, e00631-16. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.; Klaere, S.; Fedrizzi, B.; Goddard, M.R. Regional microbial signatures positively correlate with differential wine phenotypes: Evidence for a microbial aspect to terroir. Sci. Rep. 2015, 5, 14233. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.G.; Schlosser, J.; Power, R.; Roberts, R.; Willwerth, J.; de Savigny, C. Magnitude and Interaction of Viticultural and Enological Effects. I. Impact of Canopy Management and Yeast Strain on Sensory and Chemical Composition of Chardonnay Musqué. Am. J. Enol. Vitic. 2007, 58, 12–24. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Cardoso, R.M.; Soares, P.M.M.; Cancela, J.J.; Pinto, J.G.; Santos, J.A. Integrated analysis of climate, soil, topography and vegetative growth in iberian viticultural regions. PLoS ONE 2014, 9, e10878. [Google Scholar] [CrossRef] [PubMed]
- Bouzas-Cid, Y.; Trigo-Córdoba, E.; Orriols, I.; Falqué, E.; Mirás-Avalos, J. Influence of Soil Management on the Red Grapevine (Vitis vinifera L.) Mencía Must Amino Acid Composition and Wine Volatile and Sensory Profiles in a Humid Region. Beverages 2018, 4, 76. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Arroyo, T.; Serrano, A.; Valero, E. Remanence and survival of commercial yeast in different ecological niches of the vineyard. FEMS Microbiol. Ecol. 2011, 77, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Jolly, N.P.; Di Gianvito, P.; Rantsiou, K.; Cocolin, L. Microbial interactions in winemaking: Ecological aspects and effect on wine quality. Trends Food Sci. Technol. 2022, 127, 99–113. [Google Scholar] [CrossRef]
- Varela, C.; Borneman, A.R. Yeasts found in vineyards and wineries. Yeast 2017, 34, 111–128. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Vélez, S.; Barajas, E.; Rubio, J.A.; Vacas, R.; Poblete-Echeverría, C. Effect of Missing Vines on Total Leaf Area Determined by NDVI Calculated from Sentinel Satellite Data: Progressive Vine Removal Experiments. Appl. Sci. 2020, 10, 3612. [Google Scholar] [CrossRef]











| (A) DO-Org/Con | Mo-Con | Mo-Org | RB-Con | RB-Org | Ri-Con | Ri-Org | RS-Con | RS-Org | ||
|---|---|---|---|---|---|---|---|---|---|---|
| W-gs.o.s.: | 0.0316 | Mo-Con | 0.3367 | 0.3334 | 0.3313 | 0.3339 | 1 | 0.3382 | 0.3356 | |
| F: | 11.00 | Mo-Org | 37.54 | 0.3270 | 0.3360 | 0.3184 | 0.3271 | 0.3359 | 0.3319 | |
| p(same): | 0.0029 | RB-Con | 40.01 | 71.67 | 1 | 0.3329 | 0.3337 | 0.3322 | 0.3310 | |
| RB-Org | 121.00 | 104.40 | 0.22 | 0.3345 | 0.3310 | 0.3315 | 0.3286 | |||
| Ri-Con | 27.84 | 58.20 | 23.22 | 83.29 | 0.3397 | 0.3299 | 0.3331 | |||
| Ri-Org | 0.27 | 3.89 | 6.60 | 7.83 | 1.29 | 0.6627 | 0.6708 | |||
| RS-Con | 16.77 | 21.10 | 6.81 | 25.96 | 3.95 | 0.53 | 1 | |||
| RS-Org | 44.94 | 26.65 | 3.30 | 13.52 | 45.15 | 0.98 | 0.98 | |||
| (B) DOs | Mo | RB | Ri | RS | ||||||
| W-gs.o.s.: | 0.1314 | Mo | 0.0279 | 0.1737 | 0.1340 | |||||
| F: | 7.76 | RB | 18.40 | 0.0275 | 0.0654 | |||||
| p(same): | 0.0046 | Ri | 2.05 | 16.81 | 0.3275 | |||||
| RS | 3.55 | 22.16 | 1.34 | |||||||
| (C) Grape varieties | Men | Trx | Alb | Bra | ||||||
| W-gs.o.s.: | 0.1314 | Men | 0.7486 | 0.1311 | 0.7985 | |||||
| F: | 0.96 | Trx | 0.17 | 0.3231 | 0.8200 | |||||
| p(same): | 0.4355 | Alb | 2.82 | 1.78 | 0.3364 | |||||
| Bra | 0.35 | 0.13 | 24.96 | |||||||
| (D) Org-Con | Org | Con | ||||||||
| W-gs.o.s.: F: p(same): | 0.3964 1.23 0.2777 | Org | 0.2855 | |||||||
| Con | 1.231 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrillo, D.; Blanco, P.; Vélez, S. Can Satellite Remote Sensing Assist in the Characterization of Yeasts Related to Biogeographical Origin? Sensors 2023, 23, 2059. https://0-doi-org.brum.beds.ac.uk/10.3390/s23042059
Castrillo D, Blanco P, Vélez S. Can Satellite Remote Sensing Assist in the Characterization of Yeasts Related to Biogeographical Origin? Sensors. 2023; 23(4):2059. https://0-doi-org.brum.beds.ac.uk/10.3390/s23042059
Chicago/Turabian StyleCastrillo, David, Pilar Blanco, and Sergio Vélez. 2023. "Can Satellite Remote Sensing Assist in the Characterization of Yeasts Related to Biogeographical Origin?" Sensors 23, no. 4: 2059. https://0-doi-org.brum.beds.ac.uk/10.3390/s23042059