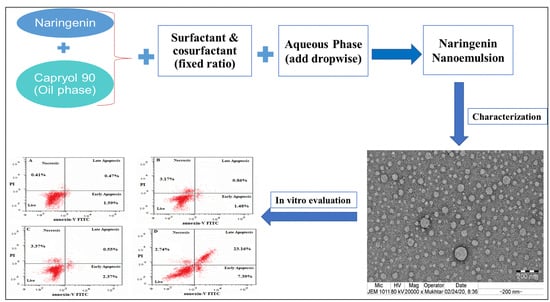
Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Solubility Studies
2.2. Formulation and Optimization of the Naringenin-Loaded Nanoemulsion
2.2.1. Mean Globule Size
2.2.2. Optimization of the Naringenin-Loaded Nano Emulsion (NAR-NE)
2.3. Thermodynamic Stability
2.4. Characterization and Evaluation of the Optimized Nanoemulsion
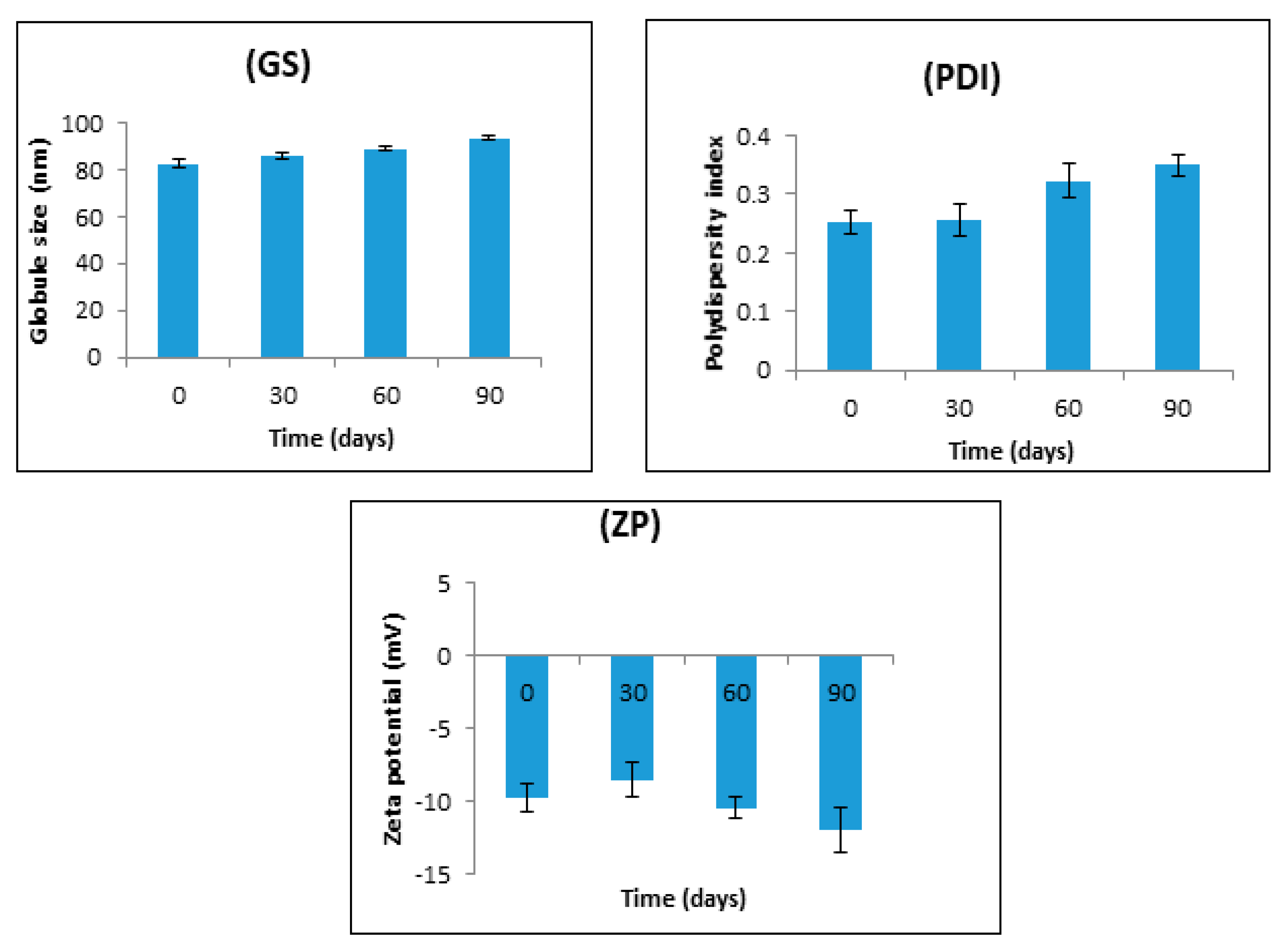
2.4.1. Mean Globule Size, PDI, and Zeta Potential
2.4.2. Transmission Electron Microscopy
2.4.3. Viscosity and Refractive Index
2.4.4. Percentage Transmittance and Drug Content
2.4.5. In Vitro Naringenin Release
2.4.6. Stability Studies
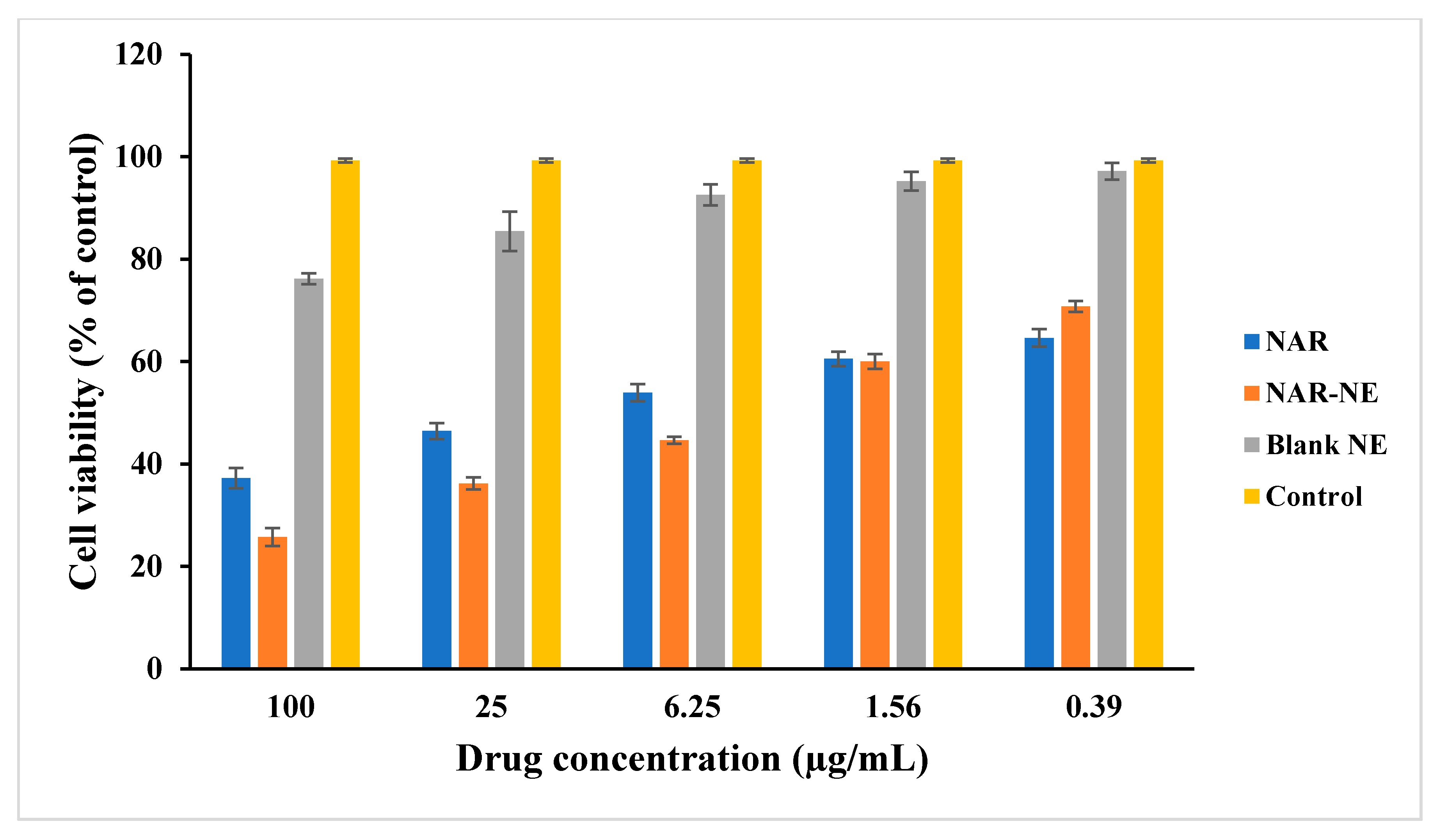
2.5. Cell Viability Using MTT Assay
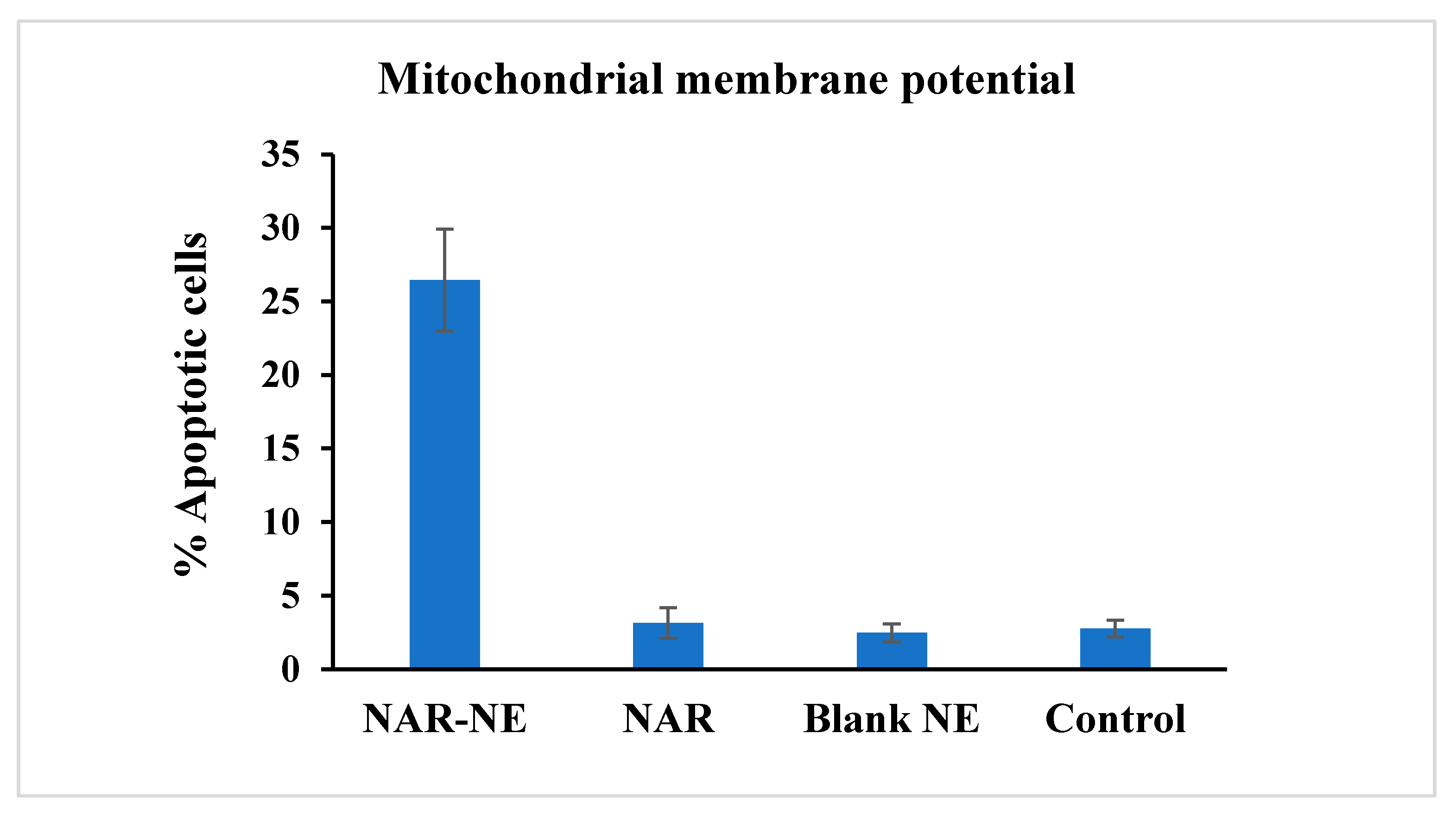
2.6. Effect of the Naringenin Nanoemulsion on Inducing Apoptosis as Detected Using the Mitochondrial Membrane Potential Method
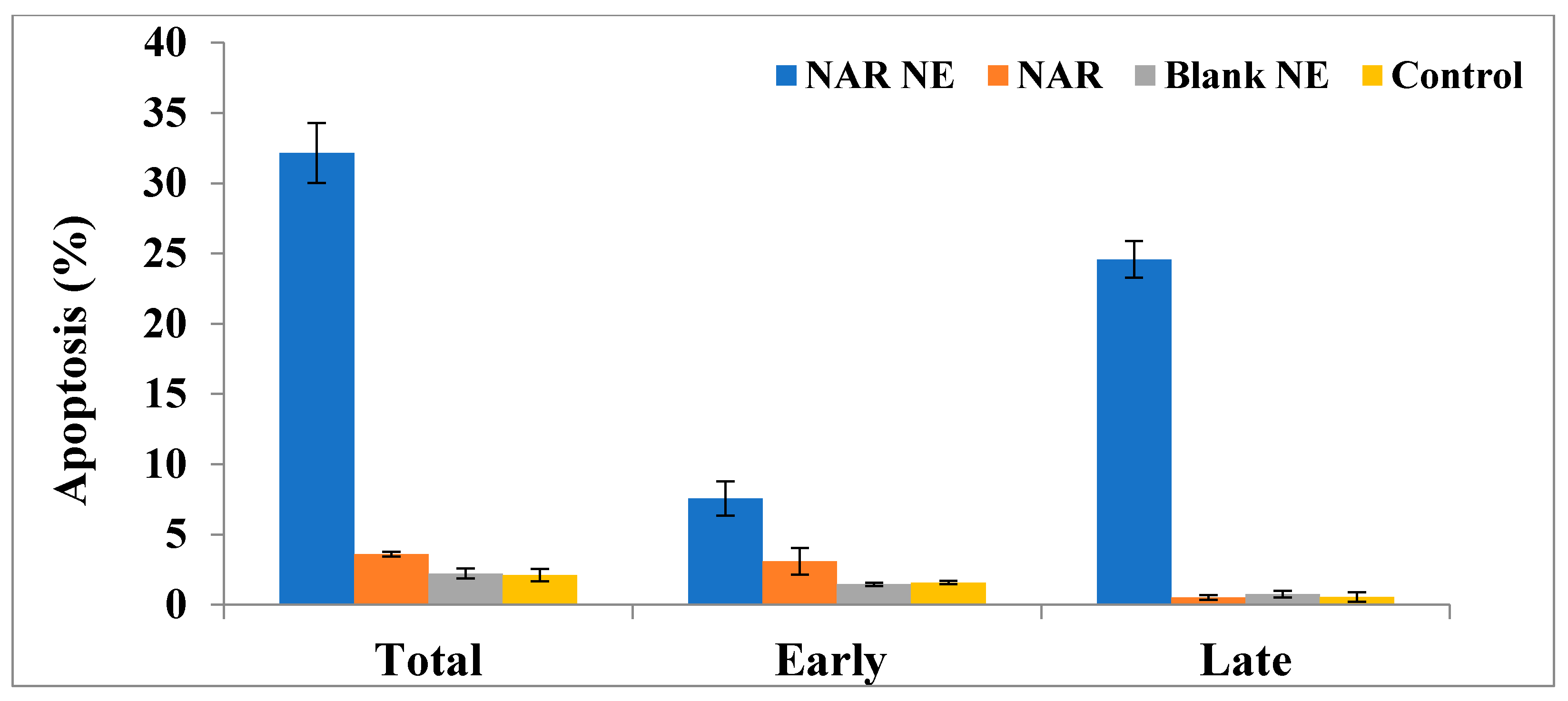
2.7. Apoptosis as Determined Using the Annexin-PI Method
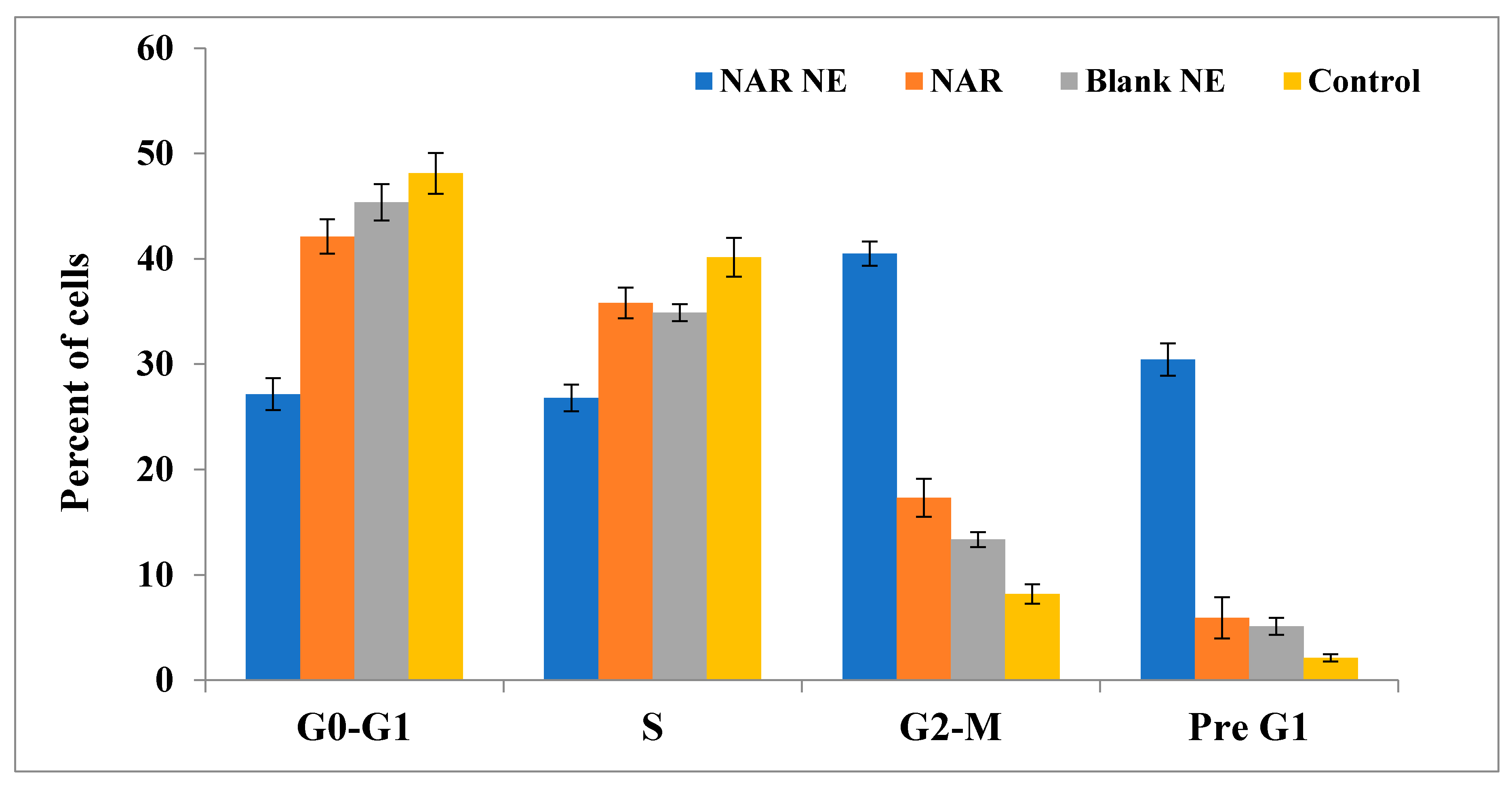
2.8. Cell Cycle Analysis by Annexin-PI Method
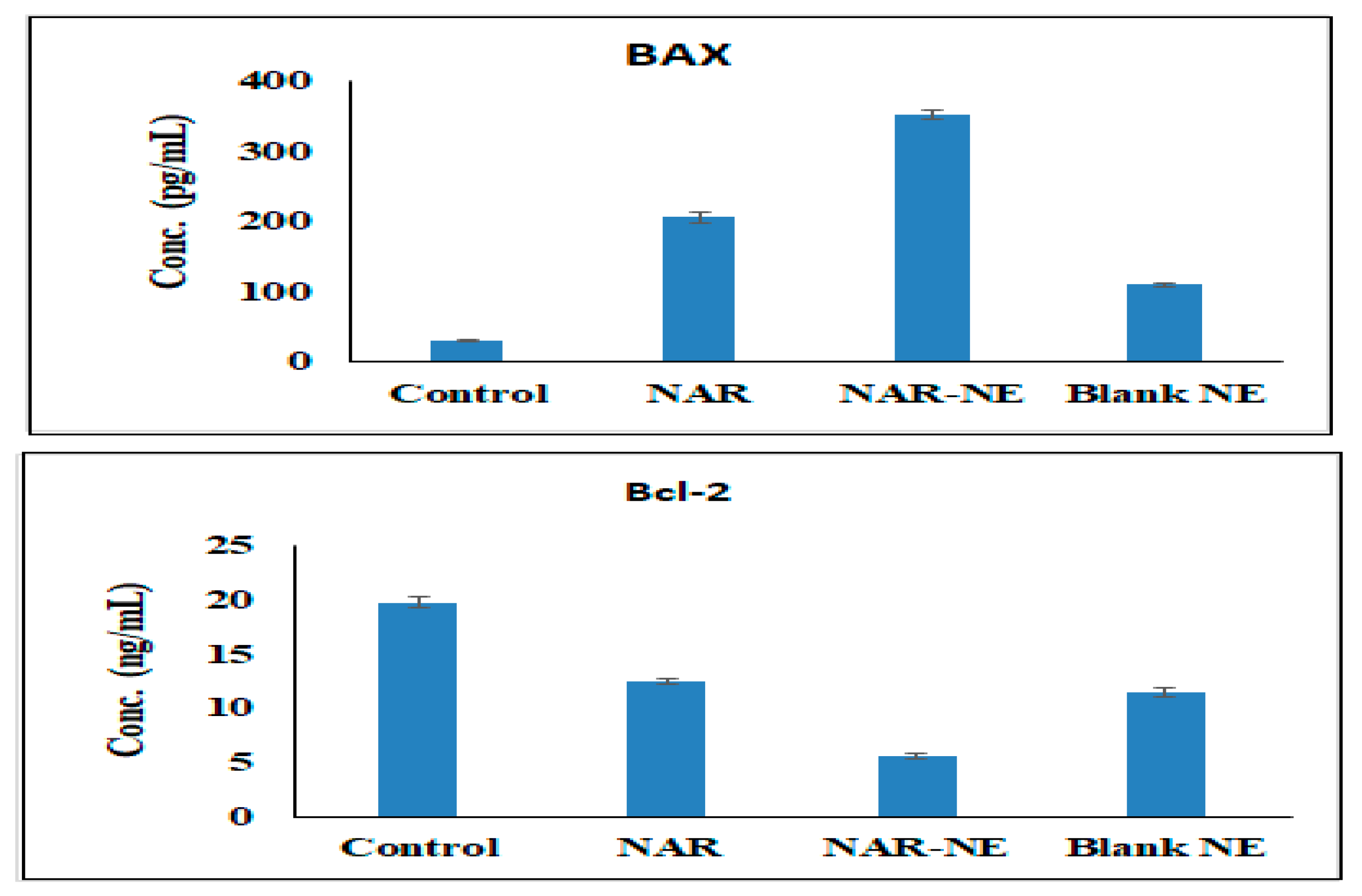
2.9. Effect of the Naringenin Nanoemulsion on the Expression of Bax and Bcl-2 Proteins
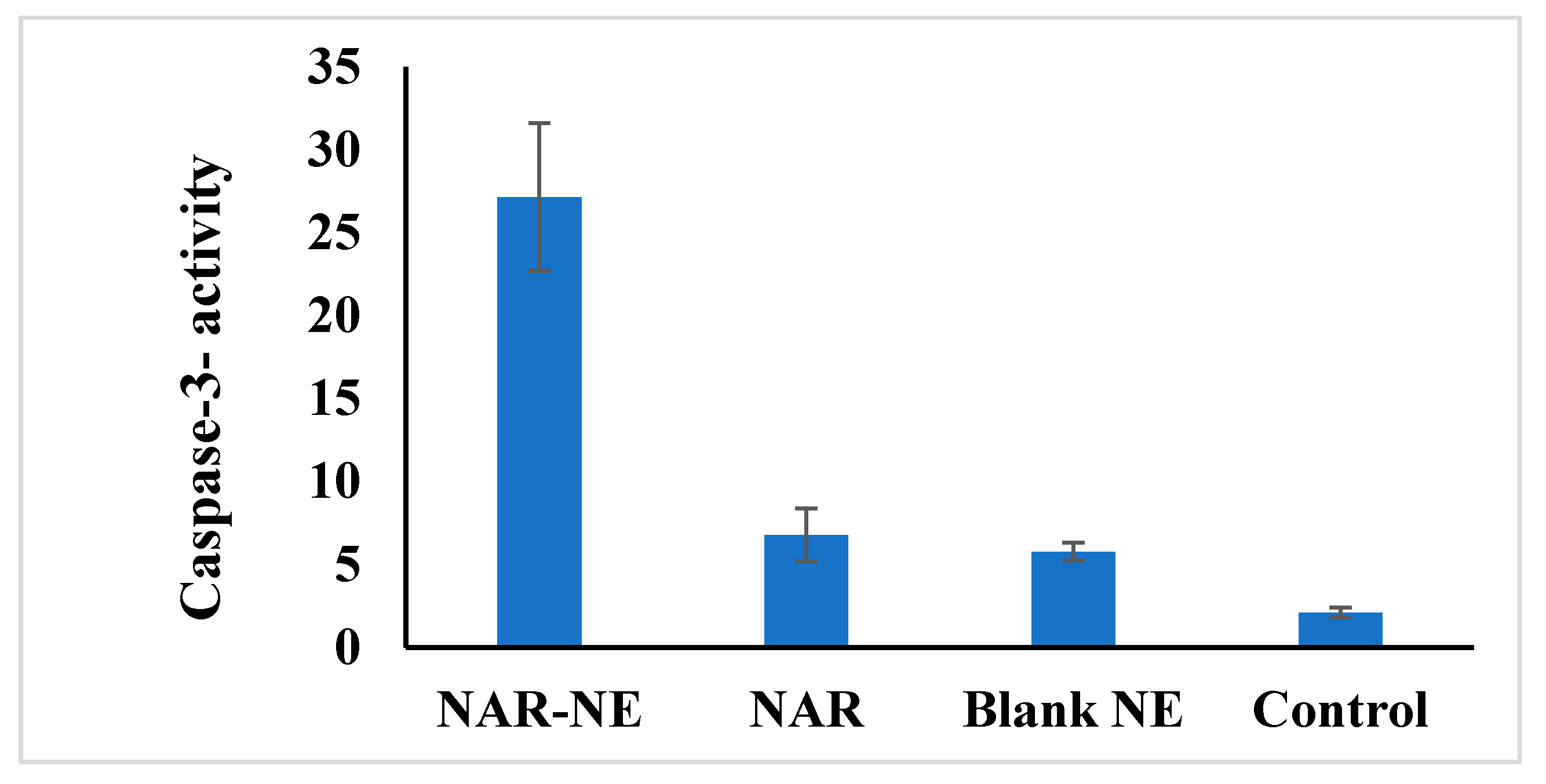
2.10. Effect of the Naringenin Nanoemulsion on Caspase-3 Activity in A549 Cells
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. Solubility Studies
3.4. Formulation and Optimization
3.4.1. Experimental Design
3.4.2. Formulation of the Naringenin-Loaded Nanoemulsions
3.4.3. Optimization of the Naringenin-Loaded Nanoemulsion
3.5. Thermodynamic Stability
3.6. Characterization and Evaluation of the Optimized Nanoemulsion
3.6.1. Transmission Electron Microscopy (TEM)
3.6.2. Viscosity and Refractive Index
3.6.3. Percent Transmittance and Drug Content
3.6.4. In Vitro Naringenin Release
3.6.5. Stability Studies
3.7. Cell Viability Determination Using MTT Assay
3.8. Mitochondrial Membrane Potential
3.9. Apoptosis Determined by the Annexin V-Propidium Iodide Method
3.10. Cell Cycle Analysis by the Annexin-PI Method
3.11. Bax and Bcl-2 Proteins Expression Estimation
3.12. Caspase-3 Analysis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aldawsari, H.M.; Gorain, B.; Alhakamy, N.A.; Md, S. Role of therapeutic agents on repolarisation of tumour-associated macrophage to halt lung cancer progression. J. Drug Target 2020, 28, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Murray, T.; Ward, E.; Samuels, A.; Tiwari, C.; Ghafoor, A.; Feuer, J.; Thun, J. Cancer statistics. CA Cancer J. Clin. 2005, 55, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Sangodkar, J.; Katz, S.; Melville, H.; Narla, G. Lung adenocarcinoma: Lessons in translation from bench to bedside. Mt. Sinai J. Med. 2010, 77, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, R.; Babu, A.; Amreddy, N.; Basalingappa, K.; Mehta, M.; Chen, A.; Zhao, Y.D.; Kompella, U.B.; Munshi, A.; Ramesh, R. Folate receptor-targeted nanoparticle delivery of HuR-RNAi suppresses lung cancer cell proliferation and migration. J. Nanobiotechnol. 2016, 14, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottaghitalab, F.; Farokhi, M.; Fatahi, Y.; Atyabi, F.; Dinarvand, R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. J. Control. Release 2019, 295, 250–267. [Google Scholar] [CrossRef]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [Green Version]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles: Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.L.; Chang, Y.M.; Lai, S.C. Naringenin inhibits migration of lung cancer cells via the inhibition of matrix metalloproteinases-2 and -9. Exp. Ther. Med. 2017, 13, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Bodduluru, L.N.; Kasala, E.R.; Madhana, R.M. Naringenin ameliorates inflammation and cell proliferation in benzo(a)pyrene induced pulmonary carcinogenesis by modulating CYP1A1, NFκB and PCNA expression. Int. Immunopharmacol. 2016, 30, 102–110. [Google Scholar] [CrossRef]
- Totta, P.; Acconcia, F.; Leone, S.; Cardillo, I.; Marino, M. Mechanisms of naringenin-induced apoptotic cascade in cancer cells: Involvement of estrogen receptor alpha and beta signalling. IUBMB Life 2004, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Arul, D.; Subramanian, P. Naringenin (citrus flavonone) induces growth inhibition, cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Yu, T.; Liu, Y.; Jiang, J.; Xu, J.; Zhao, Y.; Hao, Y.; Qiu, Y.; Zhao, W.; Wu, C. Naringenin-loaded solid lipid nanoparticles: Preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des. Dev. Ther. 2016, 1, 911–925. [Google Scholar]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Raeisi, S.; Chavoshi, H.; Mohammadi, M.; Ghorbani, M.; Sabzichi, M.; Ramezani, F. Naringenin-loaded nano-structured lipid carrier fortifies oxaliplatin-dependent apoptosis in HT-29 cell line. Process Biochem. 2019, 83, 168–175. [Google Scholar] [CrossRef]
- Chaurasia, S.; Patel, R.R.; Vure, P.; Mishra, B. Potential of Cationic-Polymeric Nanoparticles for Oral Delivery of Naringenin: In Vitro and In Vivo Investigations. J. Pharm. Sci. 2018, 107, 706–716. [Google Scholar] [CrossRef]
- Sumathi, R.; Tamizharasi, S.; Sivakumar, T. Formulation and evaluation of polymeric nanosuspension of naringenin. Int. J. App. Pharm. 2017, 9, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Akhter, M.H.; Kumar, S.; Nomani, S. Sonication tailored enhance cytotoxicity of naringenin nanoparticle in pancreatic cancer: Design, optimization, and in vitro studies. Drug Dev. Ind. Pharm. 2020, 46, 659–672. [Google Scholar] [CrossRef]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.T.R. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Ahmad, F.J.; Ahmad, W.; Alam, M.A.; Amir, M.; Ali, A. Poloxamer-chitosan-based Naringenin nanoformulation used in brain targeting for the treatment of cerebral ischemia. Saudi J. Biol. Sci. 2020, 27, 500–517. [Google Scholar] [CrossRef]
- Fuster, M.G.; Carissimi, G.; Montalbán, M.G.; Víllora, G. Improving Anticancer Therapy with Naringenin-Loaded Silk Fibroin Nanoparticles. Nanomaterials 2020, 10, 718. [Google Scholar] [CrossRef] [Green Version]
- Wan, K.; Sun, L.; Hu, X. Novel nanoemulsion based lipid nanosystems for favorable in vitro and in vivo characteristics of curcumin. Int. J. Pharm. 2016, 504, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bhardwaj, M.; Shukla, S. Carvacrol encapsulated nanocarrier/ nanoemulsion abrogates angiogenesis by downregulating COX-2, VEGF and CD31 in vitro and in vivo in a lung adenocarcinoma model. Colloids Surf. B Biointerfaces 2019, 181, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Gaba, B.; Khan, T.; Haider, M.; Alam, T.; Baboota, S.; Parvez, S.; Ali, J. Vitamin E Loaded Naringenin Nanoemulsion via Intranasal Delivery for the Management of Oxidative Stress in a 6-OHDA Parkinson’s Disease Model. BioMed Res. Int. 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Khan, A.W.; Pramod, K.; Ansari, S.H.; Sharma, R.; Ali, J. Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2012, 9, 585–598. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.; Hawc, Y.; Hoc, C.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against β-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef]
- Sandhu, S.; Kumar, R.; Beg, S.; Jain, S.; Kushwah, V.; Katare, P.; Singh, B. Natural lipid enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: Systematic approach for improved breast cancer therapeutics. Nanomedicine 2017, 13, 1703–1713. [Google Scholar] [CrossRef]
- Tsai, M.J.; Huang, Y.B.; Fang, J.W.; Fu, Y.S.; Wu, P.C. Preparation and evaluation of submicron-carriers for naringenin topical application. Int. J. Pharm. 2015, 481, 84–90. [Google Scholar] [CrossRef]
- Kale, S.N.; Deore, S.L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2016, 8, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Panner Selvam, R.; Kulkarni, P.K.; Naga Sravan Kumar Varma, V. Porous polystyrene spheres loaded self nano-emulsifying systems of rosuvastatin calcium. RSC Adv. 2015, 5, 69642–69650. [Google Scholar]
- Mayer, S.; Weiss, J.; McClements, D.J. Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: Factors influencing droplet size and stability. J. Colloid Interface Sci. 2013, 402, 122–130. [Google Scholar] [CrossRef]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef]
- Gradzielski, M. Effect of the Cosurfactant Structure on the Bending Elasticity in Nonionic Oil-in-Water Microemulsions. Langmuir 1998, 14, 6037–6044. [Google Scholar] [CrossRef]
- Tiwari, R.; Dubey, V.; Kesavan, K. Ocular Self-Microemulsifying Drug Delivery System of Prednisolone Improves Therapeutic Effectiveness in the Treatment of Experimental Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.A.; Kelmann, R.G.; Araújo, B.V.; Finatto, R.B.; Teixeira, H.F.; Koester, L.S. Development and characterization of parenteral nanoemulsions containing thalidomide. Eur. J. Pharm. Sci. 2011, 42, 238–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belhaj, N.; Dupuis, F.; Arab-Tehrany, E.; Denis, F.M.; Paris, C.; Lartaud, I.; Linder, M. Formulation, characterization and pharmacokinetic studies of coenzyme Q10 PUFA’s nanoemulsions. Eur. J. Pharm. Sci. 2012, 47, 305–312. [Google Scholar] [CrossRef]
- Harun, S.N.; Nordin, S.A.; Abd Gani, S.S.; Shamsuddin, A.F.; Basri, M.; Basri, H.B. Development of nanoemulsion for efficient brain parenteral delivery of cefuroxime: Designs, characterizations, and pharmacokinetics. Int. J. Nanomed. 2018, 13, 2571–2584. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shang, Z.; Gao, C.; Du, M.; Xu, S.; Song, H.; Liu, T. Nanoemulsion for solubilization, stabilization, and in vitro release of pterostilbene for oral delivery. AAPS Pharm. Sci. Tech. 2014, 15, 1000–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Costa, S.; Basri, M.; Shamsudin, N.; Basri, H. Stability of Positively Charged Nanoemulsion Formulation Containing Steroidal Drug for Effective Transdermal Application. J. Chem. 2014. [Google Scholar] [CrossRef] [Green Version]
- Siew Yong, T.; Lee, S.; Ong, H.; Ong, C.; Gan, S.N.; Rathbone, M.; Coombes, A. Evaluation of Biosourced Alkyd Nanoemulsions as Drug Carriers. J. Nanomater. 2015. [Google Scholar] [CrossRef] [Green Version]
- Faramarzi, L.; Dadashpour, M.; Sadeghzadeh, H.; Mahdavi, M.; Zarghami, N. Enhanced anti proliferative and pro-apoptotic effects of metformin encapsulated PLGA-PEG nanoparticles on SKOV3 human ovarian carcinoma cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Rajamani, S.; Radhakrishnan, A.; Sengodan, T.; Thangavelu, S. Augmented anticancer activity of naringenin-loaded TPGS polymeric nanosuspension for drug resistive MCF-7 human breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 1752–1761. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Md, S. Repurposing Itraconazole Loaded PLGA Nanoparticles for Improved Antitumor Efficacy in Non-Small Cell Lung Cancers. Pharmaceutics 2019, 11, 685. [Google Scholar] [CrossRef] [Green Version]
- Alhakamy, N.; Fahmy, U.; Badr-Eldin, S.; Ahmed, O.; Asfour, H.; Aldawsari, H.; Algandaby, M.; Eid, B.; Abdel-Naim, A.; Awan, Z.; et al. Optimized Icariin Phytosomes Exhibit Enhanced Cytotoxicity and Apoptosis-Inducing Activities in Ovarian Cancer Cells. Pharmaceutics 2020, 12, 346. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Kotta, S.; Ansari, S.; Sharma, R.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V.; Kumar, K.J.; Muralidharan, S.; Parasuraman, S.; Raj, P.V.; Kumar, K.V. Optimization and in-vivo evaluation of isradipine nanoparticles using Box-Behnken design surface response methodology. Open Nano 2016, 1, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Formulation of nanoemulsion: A comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015, 22, 455–466. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.; Zhang, P.; Liu, T.; Zhou, L.; Yang, G.; Lin, R.; Zhang, J. Solubilities of Naringin and Naringenin in Different Solvents and Dissociation Constants of Naringenin. J. Chem. Eng. Data 2015, 60, 932–940. [Google Scholar] [CrossRef]


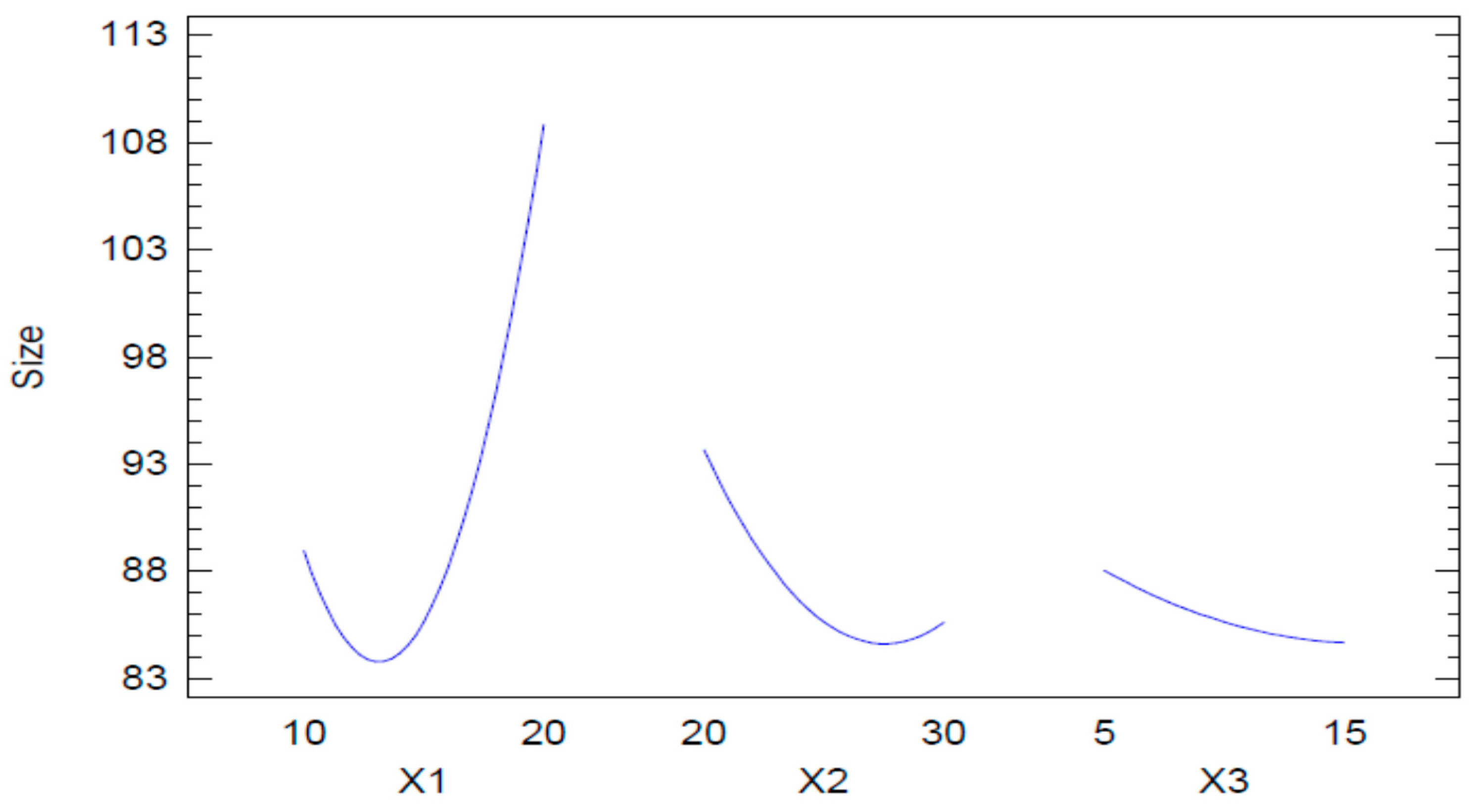

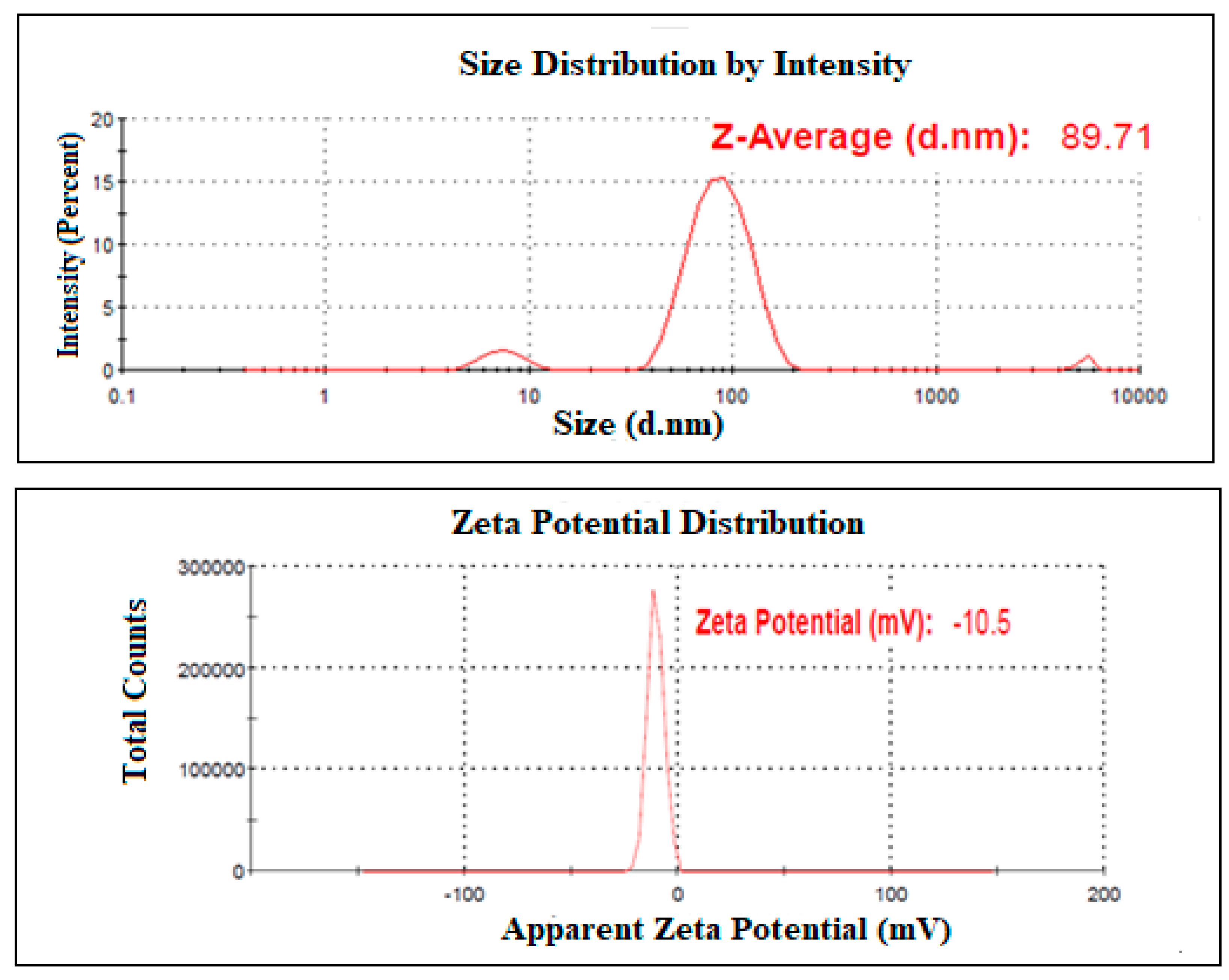
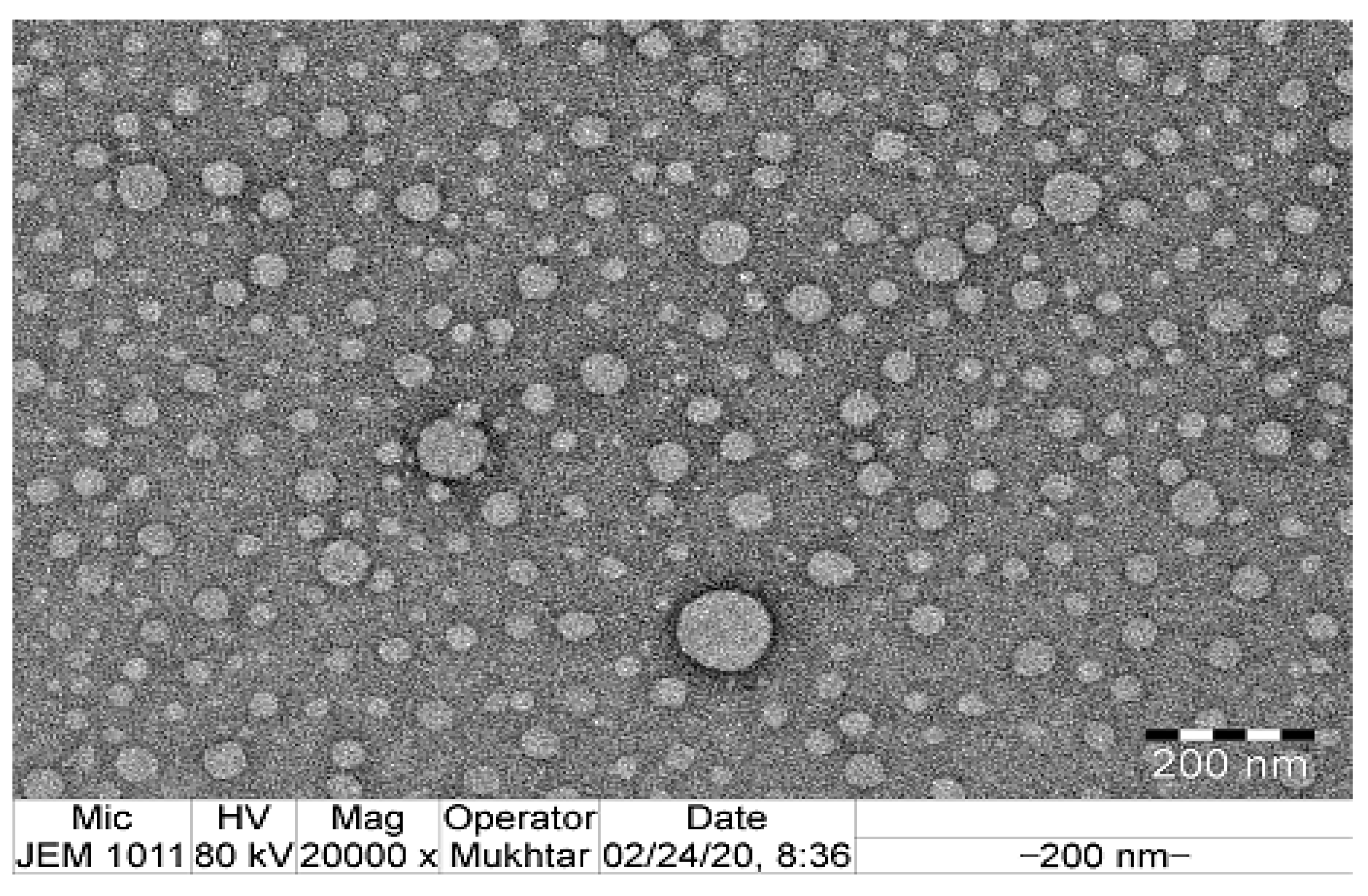


| Run | Values of the Independent Variables | Dependent Variable | ||
|---|---|---|---|---|
| Factor X1 (% v/v) | Factor X2 (% v/v) | Factor X3 (% v/v) | Mean Globule Size, GS (nm) | |
| 1 | 15 | 25 | 10 | 85.63 |
| 2 | 20 | 25 | 15 | 103.07 |
| 3 | 20 | 20 | 10 | 105.16 |
| 4 | 10 | 30 | 10 | 75.98 |
| 5 | 15 | 20 | 15 | 92.39 |
| 6 | 10 | 25 | 5 | 79.47 |
| 7 | 10 | 20 | 10 | 110.69 |
| 8 | 20 | 25 | 5 | 116.78 |
| 9 | 15 | 25 | 10 | 85.63 |
| 10 | 20 | 30 | 10 | 119.77 |
| 11 | 15 | 25 | 10 | 85.63 |
| 12 | 10 | 25 | 15 | 99.17 |
| 13 | 15 | 30 | 5 | 96.09 |
| 14 | 15 | 20 | 5 | 94.38 |
| 15 | 15 | 30 | 15 | 78.57 |
| Independent Variable Factor | Levels | |||
| Low (−1) | Medium (0) | High (1) | ||
| Concentration of Capryol 90 (X1) | 10% | 15% | 20% | |
| Concentration of Tween 20 (X2) | 20% | 25% | 30% | |
| Concentration of PEG 200 (X3) | 5% | 10% | 15% | |
| Source | Sum of Squares | Df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A:X1 | 789.435 | 1 | 789.435 | 43.68 | 0.0012 |
| B:X2 | 129.659 | 1 | 129.659 | 7.17 | 0.0439 |
| C:X3 | 22.7925 | 1 | 22.7925 | 1.26 | 0.3125 |
| AA | 649.577 | 1 | 649.577 | 35.94 | 0.0019 |
| AB | 608.116 | 1 | 608.116 | 33.65 | 0.0021 |
| AC | 278.946 | 1 | 278.946 | 15.43 | 0.0111 |
| BB | 59.1631 | 1 | 59.1631 | 3.27 | 0.1302 |
| BC | 60.3211 | 1 | 60.3211 | 3.34 | 0.1273 |
| CC | 1.93408 | 1 | 1.93408 | 0.11 | 0.7568 |
| Total error | 90.371 | 5 | 18.0742 | --- | --- |
| Total (corr.) | 2663.23 | 14 | --- | --- | --- |
| Run | Mean Globule Size (nm) | |
|---|---|---|
| Observed Values | Fitted Values | |
| 1 | 85.6333 | 85.6333 |
| 2 | 103.073 | 99.5158 |
| 3 | 105.16 | 104.53 |
| 4 | 75.98 | 76.6104 |
| 5 | 92.3933 | 96.5812 |
| 6 | 79.4667 | 83.0242 |
| 7 | 110.693 | 109.322 |
| 8 | 116.777 | 119.593 |
| 9 | 85.6333 | 85.6333 |
| 10 | 119.767 | 121.138 |
| 11 | 85.6333 | 85.6333 |
| 12 | 99.1667 | 96.35 |
| 13 | 96.0933 | 91.9054 |
| 14 | 94.3767 | 92.1904 |
| 15 | 78.5767 | 80.7629 |
| Factor | Optimized Formula/Predicted Response | |
|---|---|---|
| Independent | X1 (%) | 15.7801 |
| X2 (%) | 30.0 | |
| X3 (%) | 15.0 | |
| Dependent | GS (nm) | 83.2564 |
| Test | Conditions | Evaluation Parameters/Criteria |
|---|---|---|
| Centrifugation | 5000 rpm; 30 min | Cracking; Creaming; Phase separation |
| HCC | 4 °C and 40 °C; 48 h in each temperature; 3 cycles | |
| FTC | −20 °C and 25 °C; 48 h in each temperature; 3 cycles |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md, S.; Alhakamy, N.A.; Aldawsari, H.M.; Husain, M.; Kotta, S.; Abdullah, S.T.; A. Fahmy, U.; Alfaleh, M.A.; Asfour, H.Z. Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceuticals 2020, 13, 152. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070152
Md S, Alhakamy NA, Aldawsari HM, Husain M, Kotta S, Abdullah ST, A. Fahmy U, Alfaleh MA, Asfour HZ. Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells. Pharmaceuticals. 2020; 13(7):152. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070152
Chicago/Turabian StyleMd, Shadab, Nabil A. Alhakamy, Hibah M. Aldawsari, Mohammad Husain, Sabna Kotta, Samaa T. Abdullah, Usama A. Fahmy, Mohamed A. Alfaleh, and Hani Z. Asfour. 2020. "Formulation Design, Statistical Optimization, and In Vitro Evaluation of a Naringenin Nanoemulsion to Enhance Apoptotic Activity in A549 Lung Cancer Cells" Pharmaceuticals 13, no. 7: 152. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13070152