Overlapping Genetic Background of Coronary Artery and Carotid/Femoral Atherosclerotic Calcification
Abstract
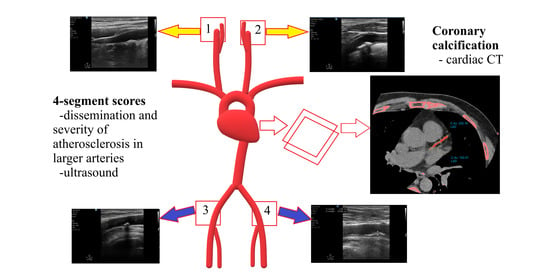
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Coronary Artery Calcification—Cardiac Non-Contrast Computed Tomography
2.3. Vascular Ultrasound
2.4. Statistics
3. Results
3.1. Descriptive Statistics
3.2. Plaque Localization
3.3. Phenotypic Correlation
3.4. Univariate Analyses
3.5. Bivariate Analysis
4. Discussion
- Coronary artery calcification’s co-occurrence with atherosclerosis in other arterial sites is less investigated. The PESA study found among 849 asymptomatic individuals that CAC co-occurred with atherosclerosis at other sites (carotid, aorta, and/or iliofemoral regions) in 89% on average [9]. Among our study participants, coronary calcification co-occurred with atherosclerotic plaques in the carotid and/or femoral arteries in 87.8% in close agreement with the previous result. About more generalized arterial calcification one study found that calcification of the superior mesenteric artery was significantly associated with the calcification of five other arterial territories (celiac trunk, coronaries, thoracic aorta, abdominal aorta, and iliac arteries) on CT [35]. Another study found that more than two-thirds of patients over 70 years old showed generalized arterial calcification in all investigated arteries (carotid, coronary, aorta, iliac arteries) and calcified atherosclerotic plaques significantly correlated in different vascular beds [36]. One-by-one carotid-coronary, femoral-coronary, and carotid-femoral atherosclerosis correlations were found to be weaker [37].
- Despite the above considerations, our study results strongly support that atherosclerotic calcification is not a passive degeneration that occurs with aging as thought earlier. It is rather an actively regulated process that also might be non-site specific (explaining why intraindividual phenotypic resemblance is high) and highly influenced by genetic predisposition. Generally, there is a growing body of evidence that microcalcification originates from extracellular vesicles released by macrophages and vascular smooth muscle cells within the plaque, which in a collagen-poor environment grow more easily into macrocalcification [50,51,52,53]. Metalloprotease enzymes, developmental, inflammatory, and metabolic factors are thought to regulate the process of atherosclerotic calcification, which in 15–20% can also turn into complex trabecular bone formation due to the plasticity of mesenchymal cells [54,55]. Calcium-phosphate imbalance is also thought to play a role, which contributes to the insufficiency of proposed calcification inhibiting pathways or manifests with the imbalance of positive and negative modulators [56,57]. However, the mechanism of atherosclerotic calcification is still not fully understood—some researchers even suggest that atheroma formation and extensive calcification might be two distinct conditions with some possible overlap [58]. Sage et al. propound that calcification might have an evolutionary explanation: it may be an ultimate immune response mechanism that develops a mechanical barrier [55].
- Screening patients with combined carotid and femoral ultrasound, detection of atherosclerotic calcification could be a cost-effective pre-selecting modality to perform cardiac CT in search for further coronary calcification similarly to the proposal of the PCV METRA group [59]. The common or overlapping genetic background of the calcification process and the hypothesized genetic predilection of plaque locations supports this idea and should generate further prospective research. Although calcified plaques are late, complicated phenotypes, nowadays a wide range of treatment options for heavily calcified plaques are already available such as the double-wire technique and rotational and orbital atherectomy [60]. Also, oral medications are being proposed to treat vascular calcification (primarily in chronic kidney disease)– however, the safety of most of these drugs needs to be addressed, especially in regard of the unwanted parallel inhibition of calcification in bones [61]. Principally, carotid-femoral ultrasound or coronary calcification assessment could help regroup patients regarding their cardiovascular risk better than traditional risk factors do alone. Our study might encourage future investigations about the genetic background of plaque dissemination “route” longitudinally and seek common genetic mechanisms that promote or reduce calcification at these localizations. We believe that a better understanding of the genetic background of atherosclerotic calcification will also lead to better therapeutic options in the future.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| CAC Severity | ||||
|---|---|---|---|---|
| 0 (n = 116) | 1–100 (Mild) (n = 36) | 100–400 (Moderate) (n = 22) | >400 (Severe) (n = 16) | |
| Localization of plaques on ultrasound: | ||||
| No plaque/4S_PL = 0/(n, %) | 62 (87.32) | 7 (9.86) | 2 (2.82) | 0 (0.00) |
| Unilateral Carotid/4S_PL = 1/(n, %) | 20 (68.97) | 7 (24.14) | 1 (3.45) | 1 (3.45) |
| Unilateral Femoral/4S_PL = 1/(n, %) | 6 (50.00) | 5 (41.67) | 1 (8.33) | 0 (0.00) |
| Unilateral Carotid and Femoral/4S_PL = 2/(n, %) | 5 (62.50) | 2 (25.00) | 1 (12.50) | 0 (0.00) |
| Bilateral Carotid/4S_PL = 2/(n, %) | 9 (42.86) | 5 (23.81) | 4 (19.05) | 3 (14.29) |
| Bilateral Femoral /4S_PL = 2/(n, %) | 1 (20.00) | 1 (20.00) | 1 (20.00) | 2 (40.00) |
| Bilateral Carotid and unilateral Femoral/4S_PL = 3/(n, %) | 6 (46.15) | 2 (15.38) | 2 (15.38) | 3 (23.08) |
| Bilateral Femoral and unilateral Carotid/4S_PL = 3/(n, %) | 6 (42.86) | 2 (14.29) | 4 (28.57) | 2 (14.29) |
| Bilateral Carotid and bilateral Femoral/4S_PL = 4/(n, %) | 1 (0.06) | 5 (29.41) | 6 (35.29) | 5 (29.41) |
| Generalized state according to ultrasound findings: | ||||
| Total plaque number (Mean Rank) | 73.03 | 111.00 | 136.95 | 163.22 |
| Total plaque number > Median (n, %) | 33 (38.37) | 20 (23.26) | 17 (19.77) | 16 (18.60) |
| Total plaque number ≤ Median (n, %) | 83 (80.58) | 16 (15.53) | 4 (3.88) | 0 (0.00) |
| 4S_PL > Median (n, %) | 27 (35.06) | 17 (22.08) | 18 (23.38) | 15 (19.48) |
| 4S_PL ≤ Median (n, %) | 89 (78.76) | 19 (16.81) | 4 (3.54) | 1 (0.88) |
| 4S_hypo > Median (n, %) | 35 (49.30) | 14 (19.72) | 10 (14.08) | 12 (16.90) |
| 4S_hypo ≤ Median (n, %) | 81 (68.07) | 22 (18.49) | 12 (10.08) | 4 (3.36) |
| 4S_mixed > Median (n, %) | 19 (31.15) | 16 (26.23) | 13 (21.31) | 13 (21.31) |
| 4S_mixed ≤ Median (n, %) | 97 (75.19) | 20 (15.50) | 9 (6.98) | 3 (2.32) |
| 4S_hyper > Median (n, %) | 24 (32.88) | 18 (24.66) | 16 (21.92) | 15 (20.55) |
| 4S_hyper(≤ Median (n, %) | 92 (78.63) | 18 (15.38) | 6 (5.13) | 1 (0.85) |
| MZ | CAC Concordants: | CAC Discordants: | ||
|---|---|---|---|---|
| MZ Twin 1+ (More Severe) | MZ Twin 2+ | MZ Twin1+ | MZ Twin 2− | |
| Mean CACS: 728.65 | CAR.bil.o. | CAR.bil.o. | CAR.bil + FEM.bil. | - |
| CACS: 1233 | CACS: 224.3 | CACS: 195.8 | - | |
| Mean CACS: 726.25 | CAR.bil.o. | CAR.bil. + FEM.uni | CAR.uni.o. | CAR.uni.o. |
| CACS: 971 | CACS: 481.5 | CACS: 19.71 | - | |
| Mean CACS: 556.86 | CAR.bil. + FEM.uni | FEM.bil. + CAR.uni | - | CAR.uni.o. |
| CACS: 822.86 | CACS: 290.87 | CACS: 10 | - | |
| Mean CACS: 533.02 | FEM.bil.o. | FEM.bil. + CAR.uni | FEM.bil. + CAR.uni | FEM.uni.o. |
| CACS: 675.83 | CACS: 390.21 | CACS: 7 | - | |
| Mean CACS: 499.885 | CAR.bil + FEM.bil. | CAR.bil.o. | CAR.bil.o. | CAR.uni + FEM.uni |
| CACS: 525.64 | CACS: 474.13 | CACS: 5.1 | - | |
| Mean CACS: 356.04 | CAR.uni.o. | CAR.bil.o. | - | - |
| CACS: 407.54 | CACS: 304.54 | CACS: 3.5 | - | |
| Mean CACS: 330.28 | CAR.bil. + FEM.uni | CAR.bil. + FEM.uni | - | CAR.Bil.o. |
| CACS: 466.98 | CACS: 193.59 | CACS: 2.5 | - | |
| Mean CACS: 319.5 | CAR.bil. + FEM.uni | CAR.bil. + FEM.bil. | CAR.bil.o. | CAR.uni. + FEM.uni |
| CACS: 360 | CACS: 279 | CACS: 2.23 | - | |
| Mean CACS: 254.95 | CAR.bil. + FEM.bil. | CAR.uni. | - | FEM.uni.o. |
| CACS: 413.58 | CACS: 96.32 | CACS: 2 | - | |
| Mean CACS: 193.35 | CAR.uni.o. | FEM.bil. + CAR.uni | - | - |
| CACS: 262.2 | CACS: 124.5 | CACS: 1.11 | - | |
| Mean CACS: 138.5 | CAR.bil. + FEM.bil. | CAR.bil. + FEM.bil. | FEM.uni.o. | - |
| CACS: 195 | CACS: 82 | CACS: 0.64 | - | |
| Mean CACS: 134.225 | CAR.bil.o. | CAR.bil.o. | ||
| CACS: 259.54 | CACS: 8.91 | |||
| Mean CACS: 124.1 | FEM.bil.o. | FEM.bil. + CAR.uni | ||
| CACS: 209 | CACS: 39.2 | |||
| Mean CACS: 71.85 | CAR.uni.o. | CAR.uni.o. | ||
| CACS: 91.88 | CACS: 51.82 | |||
| Mean CACS: 68.465 | CAR.bil.o. | CAR.uni. | ||
| CACS: 80.7 | CACS: 56.23 | |||
| Mean CACS: 39.5 | FEM.uni.o. | FEM.uni.o. | ||
| CACS: 78 | CACS: 1 | |||
| Mean CACS: 36 | CAR.Bil. + FEM.bil. | FEM.uni. | ||
| CACS: 68.19 | CACS: 3.81 |
| MZ | 0 CAC Concordants | 0 CAC Concordants | ||
|---|---|---|---|---|
| 0 US Concordants | ||||
| MZ twin 1 (+) | MZ twin 2 (+/−) | MZ twin 1 (−) | MZ twin 2 (−) | |
| CAR.uni | - | - | - | |
| CAR.uni. + FEM.uni | - | - | - | |
| CAR.uni | CAR.uni | - | - | |
| CAR.uni | - | - | - | |
| CAR.bil.+ FEM.uni. | FEM.uni.o. | - | - | |
| CAR.bil.o. | CAR.uni. | - | - | |
| CAR.uni. | - | - | - | |
| CAR.bil.o. | CAR.bil.o. | - | - | |
| CAR.uni. | CAR.uni. | - | - | |
| FEM.Bil. | FEM.uni. | - | - | |
| CAR.uni | - | - | - | |
| CAR.bil. | CAR.uni. | - | - | |
| CAR.bil. | CAR.uni. | - | - | |
| CAR.bil. | - | - | - | |
| CAR.uni. | - | |||
| CAR.bil. + FEM.uni. | CAR.uni. + FEM.uni. | |||
| CAR.uni. | - | |||
| CAR.bil. + FEM.uni. | - | |||
| CAR.uni | CAR.uni | |||
| CAR.bilat.fem.uni. | - | |||
| FEM.uni. | - | |||
| DZ | CAC Concordants: | CAC Discordants: | ||
|---|---|---|---|---|
| DZ Twin 1+ (More Severe) | DZ Twin 2+ | DZ Twin 1+ | DZ Twin 2− | |
| Mean CACS: 990.3 | CAR.bil. + FEM.bil. | FEM.bil. + CAR.uni | FEM.bil. + CAR.uni | CAR.uni + FEM.uni |
| CACS: 1248 | CACS: 732.6 | CACS: 728.44 | - | |
| Mean CACS: 877.5 | CAR.bil. + FEM.bil | CAR.uni + FEM.uni | CAR.bil. + FEM.bil. | FEM.bil. + CAR.uni |
| CACS: 1467 | CACS: 288 | CACS: 334.4 | - | |
| Mean CACS: 252.85 | FEM.bil.o. | CAR.bil. + FEM.bil. | CAR.bil. + FEM.bil. | CAR.uni |
| CACS: 413.4 | CACS: 92.3 | CACS: 202 | - | |
| Mean CACS: 221.65 | CAR.bil + FEM.bil. | CAR.uni + FEM.uni | CAR.bil.o. | - |
| CACS: 438.21 | CACS: 5.09 | CACS: 196 | - | |
| Mean CACS: 170.1 | CAR.bil + FEM.bil. | CAR.bil. + FEM.bil. | - | - |
| CACS: 256.92 | CACS: 83.25 | CACS: 189.3 | - | |
| Mean CACS: 86.46 | FEM.uni | CAR.uni | - | CAR.uni |
| CACS: 149.25 | CACS: 23.68 | CACS: 145.6 | - | |
| Mean CACS: 73.2 | CAR.bil. + FEM.bil. | CAR.bil. + FEM.uni. | FEM.bil.o. | FEM.bil. + CAR.uni |
| CACS: 79.63 | CACS: 66.76 | CACS: 62.69 | - | |
| Mean CACS: 68.74 | FEM.bil. + CAR.uni. | CAR.bil. + FEM.uni | CAR.uni. | CAR.bilat + FEM.uni |
| CACS: 114.91 | CACS: 22.57 | CACS: 61 | - | |
| CAR.uni + FEM.uni | - | |||
| CACS: 14.34 | - | |||
| - | - | |||
| CACS: 14 | - | |||
| FEM.uni | - | |||
| CACS: 11 | - | |||
| CAR.bil.o. | FEM.bil. + CAR.uni | |||
| CACS: 6.36 | - | |||
| - | CAR.bil. + FEM.uni | |||
| CACS: 3.02 | - |
| DZ | 0 CAC Concordants | 0 CAC Concordants | ||
|---|---|---|---|---|
| 0 US Concordants | ||||
| DZ Twin 1 | DZ Twin 2 | DZ Twin 1 | DZ Twin 2 | |
| CAR.bil.o. | - | - | - | |
| CAR.uni. | - | - | - | |
| FEM.bil. + CAR.uni | CAR.bil. + FEM.bil. | - | - | |
| FEM.uni | - | - | - | |
| FEM.bil. + CAR.uni | - | - | - | |
| FEM.bil.o. | CAR.uni. + FEM. Uni | - | - | |
References
- Koulaouzidis, G.; Jenkins, P.J.; Mcarthur, T. Prevalence and Distribution of Coronary Artery Calcification in Asymptomatic British Population. Can. J. Cardiol. 2013, 29, 131.e3. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.M.; Tang, W.; Detrano, R.C. Racial Differences in the Significance of Coronary Calcium in Asymptomatic Black and White Subjects with Coronary Risk Factors. J. Am. Coll. Cardiol. 1999, 34, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Demer, L.L.; Tintut, Y. Inflammatory, Metabolic, and Genetic Mechanisms of Vascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavousi, M.; Desai, C.S.; Ayers, C.; Blumenthal, R.S.; Budoff, M.J.; Mahabadi, A.-A.; Ikram, M.A.; Lugt, A.V.D.; Hofman, A.; Erbel, R.; et al. Prevalence and Prognostic Implications of Coronary Artery Calcification in Low-Risk Women. JAMA 2016, 316, 2126. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; Mcevoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Blaha, M.J.; Cainzos-Achirica, M.; Dardari, Z.; Blankstein, R.; Shaw, L.J.; Rozanski, A.; Rumberger, J.A.; Dzaye, O.; Michos, E.D.; Berman, D.S.; et al. All-Cause and Cause-Specific Mortality in Individuals with Zero and Minimal Coronary Artery Calcium: A Long-Term, Competing Risk Analysis in the Coronary Artery Calcium Consortium. Atherosclerosis 2020, 294, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naghavi, M. Asymptomatic Atherosclerosis Pathophysiology, Detection and Treatment; Humana Press: New York, NY, USA, 2010; pp. 343–345, 544. [Google Scholar] [CrossRef]
- Fernández-Friera, L.; Peñalvo, J.L.; Fernández-Ortiz, A.; Ibañez, B.; López-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; Vega, V.M.D.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.J.; Bindeman, J.; Le, T.P.; Bauer, K.; Byrd, C.; Feuerstein, I.M.; Wu, H.; O’Malley, P.G. Progression of Calcified Coronary Atherosclerosis: Relationship to Coronary Risk Factors and Carotid Intima-Media Thickness. Atherosclerosis 2008, 197, 339–345. [Google Scholar] [CrossRef]
- Chambless, L.E.; Heiss, G.; Folsom, A.R.; Rosamond, W.; Szklo, M.; Sharrett, A.R.; Clegg, L.X. Association of Coronary Heart Disease Incidence with Carotid Arterial Wall Thickness and Major Risk Factors: The Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am. J. Epidemiol. 1997, 146, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Eliasziw, M.; Dicicco, M.; Hackam, D.G.; Galil, R.; Lohmann, T. Carotid Plaque Area: A Tool for Targeting and Evaluating Preventive Vascular Therapy. Stroke 2002, 33, 2916–2922. [Google Scholar] [CrossRef] [PubMed]
- Jeevarethinam, A.; Venuraju, S.; Dumo, A.; Ruano, S.; Mehta, V.S.; Rosenthal, M.; Nair, D.; Cohen, M.; Darko, D.; Lahiri, A.; et al. Relationship between Carotid Atherosclerosis and Coronary Artery Calcification in Asymptomatic Diabetic Patients: A Prospective Multicenter Study. Clin. Cardiol. 2017, 40, 752–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protogerou, A.D.; Fransen, J.; Zampeli, E.; Argyris, A.A.; Aissopou, E.; Arida, A.; Konstantonis, G.D.; Tentolouris, N.; Makrilakis, K.; Psichogiou, M.; et al. The Additive Value of Femoral Ultrasound for Subclinical Atherosclerosis Assessment in a Single Center Cohort of 962 Adults, Including High Risk Patients with Rheumatoid Arthritis, Human Immunodeficiency Virus Infection and Type 2 Diabetes Mellitus. PLoS ONE 2015, 10, e0132307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocyigit, D.; Gurses, K.M.; Taydas, O.; Poker, A.; Ozer, N.; Hazirolan, T.; Tokgozoglu, L. Role of Femoral Artery Ultrasound Imaging in Cardiovascular Event Risk Prediction in a Primary Prevention Cohort at a Medium-Term Follow-Up. J. Cardiol. 2020, 75, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G. Carotid and Femoral Ultrasound Morphology Screening and Cardiovascular Events in Low Risk Subjects: A 10-Year Follow-up Study (the CAFES-CAVE Study). Atherosclerosis 2001, 156, 379–387. [Google Scholar] [CrossRef]
- Laclaustra, M.; Casasnovas, J.A.; Fernández-Ortiz, A.; Fuster, V.; León-Latre, M.; Jiménez-Borreguero, L.J.; Pocovi, M.; Hurtado-Roca, Y.; Ordovas, J.M.; Jarauta, E.; et al. Femoral and Carotid Subclinical Atherosclerosis Association with Risk Factors and Coronary Calcium. J. Am. Coll. Cardiol. 2016, 67, 1263–1274. [Google Scholar] [CrossRef]
- Jarauta, E.; Laclaustra, M.; Villa-Pobo, R.; Langarita, R.; Marco-Benedi, V.; Bea, A.M.; León-Latre, M.; Casasnovas, J.A.; Civeira, F. Three Dimensional Carotid and Femoral Ultrasound Is Not Superior to Two Dimensional Ultrasound as a Predictor of Coronary Atherosclerosis Among Men With Intermediate Cardiovascular Risk. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colledanchise, K.N.; Mantella, L.E.; Bullen, M.; Hétu, M.-F.; Abunassar, J.G.; Johri, A.M. Combined Femoral and Carotid Plaque Burden Identifies Obstructive Coronary Artery Disease in Women. J. Am. Soc. Echocardiogr. 2020, 33, 90–100. [Google Scholar] [CrossRef]
- Kröger, K.; Lehmann, N.; Rappaport, L.; Perrey, M.; Sorokin, A.; Budde, T.; Heusch, G.; Jöckel, K.-H.; Thompson, P.D.; Erbel, R.; et al. Carotid and Peripheral Atherosclerosis in Male Marathon Runners. Med. Sci. Sports Exerc. 2011, 43, 1142–1147. [Google Scholar] [CrossRef]
- Levula, M.; Oksala, N.; Airla, N.; Zeitlin, R.; Salenius, J.-P.; Järvinen, O.; Venermo, M.; Partio, T.; Saarinen, J.; Somppi, T.; et al. Genes Involved in Systemic and Arterial Bed Dependent Atherosclerosis—Tampere Vascular Study. PLoS ONE 2012, 7, e33787. [Google Scholar] [CrossRef] [Green Version]
- Lucatelli, P.; Fagnani, C.; Tarnoki, A.D.; Tarnoki, D.L.; Sacconi, B.; Fejer, B.; Stazi, M.A.; Salemi, M.; Cirelli, C.; D’Adamo, A.; et al. Genetic Influence on Femoral Plaque and Its Relationship with Carotid Plaque: An International Twin Study. Int. J. Cardiovasc. Imaging 2017, 34, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Tárnoki, D.L.; Tárnoki, Á.D.; Horváth, T.; Jermendy, Á.L.; Kolossváry, M.; Szilveszter, B.; Voros, V.; Kovács, A.; Molnár, A.Á.; et al. Rationale, Design, and Methodological Aspects of the BUDAPEST-GLOBAL Study (Burden of Atherosclerotic Plaques Study in Twins-Genetic Loci and the Burden of Atherosclerotic Lesions). Clin. Cardiol. 2015, 38, 699–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarnoki, A.D.; Tarnoki, D.L.; Forgo, B.; Szabo, H.; Melicher, D.; Metneki, J.; Littvay, L. The Hungarian Twin Registry Update: Turning From a Voluntary to a Population-Based Registry. Twin Res. Hum. Genet. 2019, 22, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of Coronary Artery Calcium Using Ultrafast Computed Tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Ono, J.; Hirai, S.; Yamakami, I.; Saeki, N.; Yamaura, A. Detection of Unstable Plaques in Patients with Carotid Stenosis Using B-Mode Ultrasonography. Interv. Neuroradiol. 2000, 6 (Suppl. 1), 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carotid Artery Plaque Composition—Relationship to Clinical Presentation and Ultrasound B-Mode Imaging. Eur. J. Vasc. Endovasc. Surg. 1995, 10, 23–30. [CrossRef]
- Neale, M.C.; Hunter, M.D.; Pritikin, J.N.; Zahery, M.; Brick, T.R.; Kirkpatrick, R.M.; Estabrook, R.; Bates, T.C.; Maes, H.H.; Boker, S.M. OpenMx 2.0: Extended Structural Equation and Statistical Modeling. Psychometrika 2015, 81, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Rijsdijk, F.V. Analytic Approaches to Twin Data Using Structural Equation Models. Brief. Bioinform. 2002, 3, 119–133. [Google Scholar] [CrossRef]
- Mcgue, M.; Bouchard, T.J. Adjustment of Twin Data for the Effects of Age and Sex. Behav. Genet. 1984, 14, 325–343. [Google Scholar] [CrossRef]
- Molnár, S.; Kerényi, L.; Ritter, M.A.; Magyar, M.T.; Ida, Y.; Szöllősi, Z.; Csiba, L. Correlations between the Atherosclerotic Changes of Femoral, Carotid and Coronary Arteries. J. Neurol. Sci. 2009, 287, 241–245. [Google Scholar] [CrossRef]
- Helck, A.; Bianda, N.; Canton, G.; Yuan, C.; Hippe, D.S.; Reiser, M.F.; Gallino, A.; Wyttenbach, R.; Saam, T. Intra-Individual Comparison of Carotid and Femoral Atherosclerotic Plaque Features with in Vivo MR Plaque Imaging. Int. J. Cardiovasc. Imaging 2015, 31, 1611–1618. [Google Scholar] [CrossRef]
- Zhao, Q.; Cai, Z.; Cai, J.; Zhao, X.; Li, F.; Yuan, C. Correlation of Coronary Plaque Phenotype and Carotid Atherosclerotic Plaque Composition. Am. J. Med Sci. 2011, 342, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Arad, Y.; Spadaro, L.A.; Roth, M.; Scordo, J.; Goodman, K.; Sherman, S.; Lledo, A.; Lerner, G.; Guerci, A.D. Correlations Between Vascular Calcification and Atherosclerosis: A Comparative Electron Beam CT Study of the Coronary and Carotid Arteries. J. Comput. Assist. Tomogr. 1998, 22, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.C.; Wright, C.M.; Criqui, M.H.; Allison, M.A. Superior Mesenteric Artery Calcification Is Associated with Cardiovascular Risk Factors, Systemic Calcified Atherosclerosis, and Increased Mortality. J. Vasc. Surg. 2018, 67, 1484–1490. [Google Scholar] [CrossRef]
- Allison, M.A.; Criqui, M.H.; Wright, C.M. Patterns and Risk Factors for Systemic Calcified Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Moreyra, E.; Moreyra, C.; Tibaldi, M.A.; Crespo, F.; Arias, V.; Lepori, A.J.; Moreyra, E.A. Concordance and Prevalence of Subclinical Atherosclerosis in Different Vascular Territories. Vascular 2020, 28, 285–294. [Google Scholar] [CrossRef]
- Peyser, P.A.; Bielak, L.F.; Chu, J.S.; Turner, S.T.; Ellsworth, D.L.; Boerwinkle, E.; Sheedy, P.F. Heritability of Coronary Artery Calcium Quantity Measured by Electron Beam Computed Tomography in Asymptomatic Adults. Circulation 2002, 106, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Bushrow, A.E.; Bielak, L.F.; Sheedy, P.F.; Turner, S.T.; Kullo, I.J.; Lin, X.; Peyser, P.A. Coronary Artery Calcification Progression Is Heritable. Circulation 2007, 116, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Tarnoki, A.D.; Baracchini, C.; Tarnoki, D.L.; Lucatelli, P.; Boatta, E.; Zini, C.; Fanelli, F.; Molnar, A.A.; Meneghetti, G.; Stazi, M.A.; et al. Evidence for a Strong Genetic Influence on Carotid Plaque Characteristics. Stroke 2012, 43, 3168–3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfohl, M.; Athanasiadis, A.; Koch, M.; Clemens, P.; Benda, N.; Häring, H.U.; Karsch, K.R. Insertion/Deletion Polymorphism of the Angiotensin I-Converting Enzyme Gene Is Associated With Coronary Artery Plaque Calcification As Assessed by Intravascular Ultrasound. J. Am. Coll. Cardiol. 1998, 31, 987–991. [Google Scholar] [CrossRef] [Green Version]
- Kardia, S.L.R.; Haviland, M.B.; Ferrell, R.E.; Sing, C.F. The Relationship Between Risk Factor Levels and Presence of Coronary Artery Calcification Is Dependent On Apolipoprotein E Genotype. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Pöllänen, P.J.; Lehtimäki, T.; Ilveskoski, E.; Mikkelsson, J.; Kajander, O.A.; Laippala, P.; Perola, M.; Goebeler, S.; Penttilä, A.; Mattila, K.M.; et al. Coronary Artery Calcification Is Related to Functional Polymorphism of Matrix Metalloproteinase 3: The Helsinki Sudden Death Study. Atherosclerosis 2002, 164, 329–335. [Google Scholar] [CrossRef]
- Farzaneh-Far, A.; Davies, J.D.; Braam, L.A.; Spronk, H.M.; Proudfoot, D.; Chan, S.-W.; O’shaughnessy, K.M.; Weissberg, P.L.; Vermeer, C.; Shanahan, C.M. A Polymorphism of the Human Matrix γ-Carboxyglutamic Acid Protein Promoter Alters Binding of an Activating Protein-1 Complex and Is Associated with Altered Transcription and Serum Levels. J. Biol. Chem. 2001, 276, 32466–32473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, S.K.; Boelte, K.C.; Barb, J.J.; Joehanes, R.; Zhao, X.; Cheng, Q.; Adams, L.; Teer, J.K.; Accame, D.S.; Chowdhury, S.; et al. Integrative DNA, RNA, and Protein Evidence Connects TREML4 to Coronary Artery Calcification. Am. J. Hum. Genet. 2014, 95, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Bos, D.; Ikram, M.A.; Isaacs, A.; Verhaaren, B.F.; Hofman, A.; Duijn, C.M.V.; Witteman, J.C.; Lugt, A.V.D.; Vernooij, M.W. Genetic Loci for Coronary Calcification and Serum Lipids Relate to Aortic and Carotid Calcification. Circ. Cardiovasc. Genet. 2013, 6, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-C.; Lloyd-Jones, D.M.; Guo, X.; Rajamannan, N.M.; Lin, S.; Du, P.; Huang, Q.; Hou, L.; Liu, K. Gene Expression Variation between African Americans and Whites Is Associated with Coronary Artery Calcification: The Multiethnic Study of Atherosclerosis. Physiol. Genom. 2011, 43, 836–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdes, A.M.; Wolfe, M.L.; O’Brien, E.J.; Spurr, N.K.; Gefter, W.; Rut, A.; Groot, P.H.; Rader, D.J. Val64Ile Polymorphism in the C-C Chemokine Receptor 2 Is Associated With Reduced Coronary Artery Calcification. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1924–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulff-Møller, C.J.; Svendsen, A.J.; Viemose, L.N.; Jacobsen, S. Concordance of Autoimmune Disease in a Nationwide Danish Systemic Lupus Erythematosus Twin Cohort. Semin. Arthritis Rheum. 2018, 47, 538–544. [Google Scholar] [CrossRef]
- New, S.E.P.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-Derived Matrix Vesicles. Circ. Res. 2013, 113, 72–77. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Davies, J.D.; Reynolds, J.L.; Mcnair, R.; Jones, G.T.; Sidibe, A.; Schurgers, L.J.; Skepper, J.N.; Proudfoot, D.; Mayr, M.; et al. Calcium Regulates Key Components of Vascular Smooth Muscle Cell–Derived Matrix Vesicles to Enhance Mineralization. Circ. Res. 2011, 109, e1–e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, H.C. Matrix Vesicles and Calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and Growth of Extracellular-Vesicle-Derived Microcalcification in Atherosclerotic Plaques. Nat. Mater. 2016, 15, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sage, A.P.; Tintut, Y.; Demer, L.L. Regulatory Mechanisms in Vascular Calcification. Nat. Rev. Cardiol. 2010, 7, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Fuery, M.A.; Liang, L.; Kaplan, F.S.; Mohler, E.R. Vascular Ossification: Pathology, Mechanisms, and Clinical Implications. Bone 2018, 109, 28–34. [Google Scholar] [CrossRef]
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has Our Understanding of Calcification in Human Coronary Atherosclerosis Progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speer, M.Y.; Giachelli, C.M. Regulation of Cardiovascular Calcification. Cardiovasc. Pathol. 2004, 13, 63–70. [Google Scholar] [CrossRef]
- Henein, M.; Nicoll, R. Atherosclerosis and Extensive Arterial Calcification: The Same Condition? Int. J. Cardiol. 2010, 141, 1–2. [Google Scholar] [CrossRef]
- Megnien, J.L.; Sene, V.; Jeannin, S.; Hernigou, A.; Plainfosse, M.C.; Merli, I.; Atger, V.; Moatti, N.; Levenson, J.; Simon, A. Coronary Calcification and Its Relation to Extracoronary Atherosclerosis in Asymptomatic Hypercholesterolemic Men. The PCV METRA Group. Circulation 1992, 85, 1799–1807. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.; Costopoulos, C.; Gorog, D.A.; Prasad, A.; Srinivasan, M. Treatment of Calcified Coronary Artery Lesions. Expert Rev. Cardiovasc. Ther. 2016, 14, 683–690. [Google Scholar] [CrossRef]
- O’neill, W.C.; Lomashvili, K.A. Recent Progress in the Treatment of Vascular Calcification. Kidney Int. 2010, 78, 1232–1239. [Google Scholar] [CrossRef] [Green Version]

| Total | MZ | DZ | P | |
|---|---|---|---|---|
| Zygosity | 190 | 120 | 70 | |
| Male (n, %) | 72 (37.89) | 48 (40) | 24 (34.29) | 0.43 |
| Age (mean, SD) | 56.84 ± 9.33 | 55.46 ± 9.75 | 59.16 ± 8.11 | 0.01 |
| BMI (kg/m2) (mean, SD) | 27.57 ± 4.65 | 27.85 ± 4.46 | 27.08 ± 4.95 | 0.27 |
| Hypertension (n, %) | 79 (41.58) | 50 (41.67) | 29 (41.43) | 0.96 |
| Diabetes (n, %) | 14 (7.37) | 10 (8.33) | 4 (5.71) | 0.52 |
| Dyslipidaemia (n, %) | 83 (43.68) | 49 (40.83) | 34 (48.57) | 0.26 |
| Smoking (n, %) | 70 (36.84) | 44 (36.67) | 26 (37.14) | 0.89 |
| Coronary plaque occurrence (CACS > 0) (n, %) | 74 (38.95) | 44 (36.67) | 30 (42.86) | 0.39 |
| Carotid plaque occurrence (n, %) | 89 (46.84) | 54 (45.00) | 35 (50.00) | 0.51 |
| Femoral plaque occurrence (n, %) | 71 (37.37) | 38 (31.67) | 33 (47.14) | 0.03 |
| Carotid/femoral and coronary plaque co-occurrence (n, %) | 65 (34.21) | 39 (32.50) | 26 (37.14) | 0.51 |
| Carotid + femoral + coronary plaque co-occurrence (all 3) (n, %) | 32 (16.84) | 16 (13.33) | 16 (22.86) | 0.09 |
| 4S_PL > 1 (n, %) 1 | 119 (62.63) | 76 (63.33) | 43 (61.43) | 0.79 |
| 4S_hypo > 1 (n, %) 2 | 71 (37.37) | 46 (38.33) | 25 (35.71) | 0.72 |
| 4S_mixed > 1 (n, %) 2 | 61 (34.21) | 39 (32.5) | 22 (31.43) | 0.88 |
| 4S_hyper > 1 (n, %) 2 | 75 (37.5) | 44 (34.9) | 31 (41.9) | 0.36 |
| 4S_mixed/hyper > 1 (n, %) 2 | 98 (51.58) | 58 (48.33) | 40 (57.14) | 0.38 |
| N = 190 | CACS | p |
|---|---|---|
| 4S_PL | 0.557 | <0.01 |
| 4S_hypo | 0.289 | <0.01 |
| 4S_mixed | 0.444 | <0.01 |
| 4S_hyper | 0.551 | <0.01 |
| 4S_mixed/hyper | 0.604 | <0.01 |
| Trait | Model | Goodness-of-Fit Indices | Parameter Estimates (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trait | Model | AIC | −2LL | df | DiffLL | p-Value | A | C | E |
| CAC | ACE | 337.2 | 311.9 | 11 | Ref. | Ref. | 0.67 (0.16, 1) | 0 (0, 0.38) | 0.33 (0, 0.67) |
| AE * | 334.6 | 311.9 | 10 | 0 | 1 | 0.67 (0.35, 1) | - | 0.33 (0, 0.65) | |
| CE | 340.1 | 317.5 | 10 | −5.5 | .02 | - | 0.43 (0.15, 0.66) | 0.57 (0.35, 0.85) | |
| E | 346.2 | 326.1 | 9 | −14.1 | .00 | - | - | 1 | |
| Sat. | 346.9 | ||||||||
| 4S_hypo | ACE | 408.7 | 378.2 | 13 | Ref. | Ref. | 0 (0, 0.41) | 0.18 (0, 0.45) | 0.82 (0.55, 1) |
| AE | 406.9 | 379.2 | 12 | −0.9 | 0.13 (0, 0.45) | - | 0.87 (0.56, 1) | ||
| CE | 406.0 | 378.2 | 12 | 0 | 1 | - | 0.18 (0, 0.45) | 0.82 (0.55, 1) | |
| E * | 404.9 | 379.7 | 11 | −1.5 | 0.47 | - | - | 1 | |
| Sat. | 419.7 | ||||||||
| 4S_mixed | ACE | 342.7 | 312.2 | 13 | Ref. | Ref. | 0.49 (0, 0.76) | 0 (0, 0.49) | 0.50 (0.24, 1) |
| AE * | 340.0 | 312.2 | 12 | 0 | 1 | 0.50 (0, 0.76) | - | 0.50 (0.24,1) | |
| CE | 342.4 | 314.6 | 12 | −2.4 | 0.13 | - | 0.32 (0.02, 0.57) | 0.68 (0.43, 0.98) | |
| E | 344.2 | 319.0 | 11 | −6.8 | 0.03 | - | - | 1 | |
| Sat. | 355.0 | ||||||||
| 4S_hyper | ACE | 363.4 | 332.9 | 13 | Ref. | Ref. | 0.69 (0.19, 1) | 0 (0.38, 1) | 0.31 (0, 0.63) |
| AE * | 360.7 | 332.9 | 12 | 0 | 1 | 0.69 (0.38, 1) | - | 0.31 (0, 0.63) | |
| CE | 366.6 | 338.8 | 12 | −5.8 | 0.02 | - | 0.41 (0.13, 0.63) | 0.59 (0.37, 0.87) | |
| E | 371.9 | 346.8 | 11 | −13.9 | 0.00 | - | - | 1 | |
| Sat. | 371.9 | ||||||||
| Traits | Adjust | Model | Model Fit (p) | Model Fit (AIC) | A | C | E |
|---|---|---|---|---|---|---|---|
| CAC and 4S_hyper | Age and sex | ACE | - | −81.9 | 0.99 | 0 | 0.01 |
| AE * | 0.98 | −87.8 | 0.86 (0.42, 1) | - | 0.14 (0, 0.58) | ||
| CE | 0.01 | −77.4 | - | 0.42 | 0.58 | ||
| E | 0 | −67.1 | - | - | 1 | ||
| Phenotypic correlation | |||||||
| All (95% CI) | MZ (95% CI) | DZ (95% CI) | |||||
| CAC and 4S_hyper | Age and sex | 0.48 (0.30, 0.63) | 0.54 (0.31, 0.72) | 0.44 (0.14, 0.68) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernyes, A.; Piroska, M.; Fejer, B.; Szalontai, L.; Szabo, H.; Forgo, B.; Jermendy, A.L.; Molnar, A.A.; Maurovich-Horvat, P.; Jermendy, G.; et al. Overlapping Genetic Background of Coronary Artery and Carotid/Femoral Atherosclerotic Calcification. Medicina 2021, 57, 252. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57030252
Hernyes A, Piroska M, Fejer B, Szalontai L, Szabo H, Forgo B, Jermendy AL, Molnar AA, Maurovich-Horvat P, Jermendy G, et al. Overlapping Genetic Background of Coronary Artery and Carotid/Femoral Atherosclerotic Calcification. Medicina. 2021; 57(3):252. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57030252
Chicago/Turabian StyleHernyes, Anita, Marton Piroska, Bence Fejer, Laszlo Szalontai, Helga Szabo, Bianka Forgo, Adam L. Jermendy, Andrea A. Molnar, Pal Maurovich-Horvat, Gyorgy Jermendy, and et al. 2021. "Overlapping Genetic Background of Coronary Artery and Carotid/Femoral Atherosclerotic Calcification" Medicina 57, no. 3: 252. https://0-doi-org.brum.beds.ac.uk/10.3390/medicina57030252