From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine
Abstract
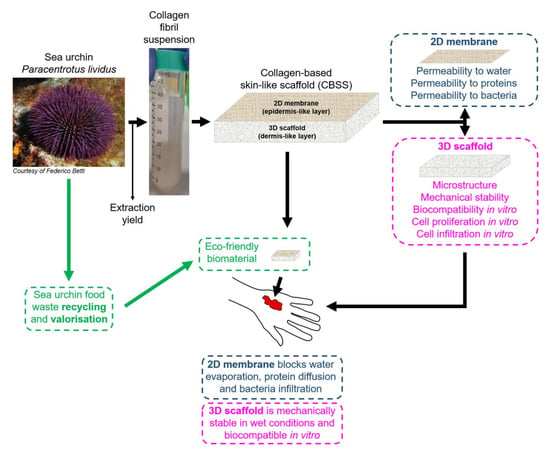
:1. Introduction
2. Results
2.1. Sea Urchin Fibrillar Collagen Extraction and Extraction Yield
2.2. 2D Collagen Membrane Permeability Tests
2.3. Bacteria Infiltration Tests
2.4. 3D Scaffold Production, Characterization and Mechanical Stability in Wet Conditions
2.5. In Vitro Tests
2.5.1. Cytotoxicity
2.5.2. Evaluation of Cell Infiltration within the 3D Scaffold
2.5.3. Evaluation of Cell Viability and Proliferation within the 3D Scaffold
3. Discussion
4. Materials and Methods
4.1. Sea Urchin Fibrillar Collagen Extraction and Extraction Yield
4.2. 2D Collagen Membrane Production
4.3. 2D Collagen Membrane Permeability Tests
4.4. Bacteria Infiltration Tests
4.5. 3D Scaffold Production
4.6. 3D Scaffold Characterization
4.7. 3D Scaffold Mechanical Stability in Wet Conditions
4.8. Cell Culture
4.9. In Vitro Tests
4.9.1. Cytotoxicity
4.9.2. Evaluation of Cell Infiltration within the 3D Scaffold
4.9.3. Evaluation of Cell Viability and Proliferation within the 3D Scaffold
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, M.E. Lower Extremity Soft Tissue & Cutaneous Plastic Surgery, In Autografts, Allografts and Xenografts in Cutaneous Surgery, 2nd ed.; Saunders Ltd.: Nottingham, UK, 2012; Volume 20, pp. 225–230. [Google Scholar]
- Shahrokhi, S.; Arno, A.; Jeschke, M.G. The use of dermal substitutes in burn surgery: Acute phase. Wound Repair Regen. 2014, 22, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, M.N.; Jeschke, M.; Amini-Nik, S. Methodologies in creating skin substitutes. Cell. Mol. Life Sci. 2016, 73, 3453–3472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Mashiko, T.; Takada, H.; Wu, S.-H.; Kanayama, K.; Feng, J.; Tashiro, K.; Asahi, R.; Sunaga, A.; Hoshi, K.; Kurisaki, A.; et al. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. J. Tissue Eng. Regen. Med. 2018, 12, 1186–1194. [Google Scholar] [CrossRef]
- Nishikimi, A.; Koyama, Y.-I.; Ishihara, S.; Kobayashi, S.; Tometsuka, C.; Kusubata, M.; Kuwaba, K.; Hayashida, O.; Hattori, S.; Katagiri, K. Collagen-derived peptides modulate CD4+ T-cell differentiation and suppress allergic responses in mice. Immun. Inflamm. Dis. 2018, 6, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Felician, F.F.; Yu, R.-H.; Li, M.-Z.; Li, C.-J.; Chen, H.-Q.; Jiang, Y.; Tang, T.; Qi, W.-Y.; Xu, H.-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol. 2019, 22, 12–20. [Google Scholar] [CrossRef]
- Pham, C.; Greenwood, J.; Cleland, H.; Woodruff, P.; Maddern, G. Bioengineered skin substitutes for the management of burns: A systematic review. Burns 2007, 33, 946–957. [Google Scholar] [CrossRef]
- Philandrianos, C.; Andrac-Meyer, L.; Mordon, S.; Feuerstein, J.M.; Sabatier, F.; Veran, J.; Magalon, G.; Casanova, D. Comparison of five dermal substitutes in full-thickness skin wound healing in a porcine model. Burns 2012, 38, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Yeon Kim, S.; Chun, T.; Byun, H.J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Barros, A.A.; Aroso, I.M.; Silva, T.H.; Mano, J.F.; Duarte, A.R.C.; Reis, R.L. Water and carbon dioxide: Green solvents for the extraction of collagen/gelatin from marine sponges. ACS Sustain. Chem. Eng. 2014, 3, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Silvipriya, K.S.; Kumar, K.K.; Bhat, A.R.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Diogo, G.S.; López-Senra, E.; Pirraco, R.P.; Canadas, R.F.; Fernandes, E.M.; Serra, J.; Pérez-Martín, R.I.; Sotelo, C.G.; Marques, A.P.; González, P.; et al. Marine collagen/apatite composite scaffolds envisaging hard tissue applications. Mar. Drugs 2018, 16, 269. [Google Scholar] [CrossRef] [Green Version]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Bhadriraju, K.; Spurlin, T.A.; Cook, R.F.; Plant, A.L. Nanomechanical properties of thin films of type I collagen fibrils. Langmuir 2010, 26, 3629–3636. [Google Scholar] [CrossRef] [PubMed]
- Bozec, L.; van der Heijden, G.; Horton, M. Collagen fibrils: Nanoscale ropes. Biophys. J. 2007, 92, 70–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, D.l.; Yannas, I.V. Recent advances in tissue synthesis in vivo by use of collagen glycosaminoglycan copolymers. Biomaterials 1996, 17, 291–299. [Google Scholar] [CrossRef]
- Di Benedetto, C.; Barbaglio, A.; Martinello, T.; Alongi, V.; Fassini, D.; Cullorà, E.; Patruno, M.; Bonasoro, F.; Barbosa, M.A.; Candia Carnevali, M.D.; et al. Production, characterization and biocompatibility of marine collagen matrices from an alternative and sustainable source: The sea urchin Paracentrotus Lividus. Mar. Drugs 2014, 12, 4912–4933. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, C.; Leggio, L.; Leone, R.; Di Benedetto, C.; Cocce’, V.; Ascagni, M.; Bonasoro, F.; La Porta, C.A.M.; Candia Carnevali, M.D.; Sugni, M. Marine-derived collagen biomaterials from echinoderm connective tissues. Mar. Environ. Res. 2017, 128, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Tricarico, S.; Barbaglio, A.; Burlini, N.; Del Giacco, L.; Ghilardi, A.; Sugni, M.; Di Benedetto, C.; Bonasoro, F.; Wilkie, I.C.; Candia Carnevali, M.D. New insights into the mutable collagenous tissue of Paracentrotus lividus: Preliminary results. Zoosymposia 2012, 7, 279–285. [Google Scholar] [CrossRef]
- Wilkie, I.C. Mutable collagenous tissue: Overview and perspectives. In Echinodermata. Progress in Molecular and Subcellular Biology. Marine Molecular Biotechnology; Matranga, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 5, pp. 221–250. [Google Scholar]
- Furesi, R.; Madau, F.A.; Pulina, P.; Sai, R.; Pinna, M.G.; Pais, A. Profitability and sustainability of edible sea urchin fishery in Sardinia (Italy). J. Coast. Conserv. 2016, 20, 299–306. [Google Scholar] [CrossRef]
- Meyer, M.; Schröpfer, M. Collagen materials—Collagen processing. Technical freedom and scientific challenges when transforming collagen into final materials. In Proceedings of the XXXII Congress of IULTCS, Istanbul, Turkey, 29–31 May 2013. [Google Scholar]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced collagen-based biomaterials for regenerative biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Debels, H.; Hamdi, M.; Abberton, K.; Morrison, W. Dermal matrices and bioengineered skin substitutes: A critical review of current options. Plast. Reconstr. Surg. Glob. Open 2015, 3, e284. [Google Scholar] [CrossRef] [PubMed]
- Addad, S.; Exposito, J.-Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). Int. J. Food Sci. Technol. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Uriarte-Montoya, M.H.; Arias-Moscoso, J.L. Jumbo squid (Dosidicus gigas) mantle collagen: Extraction, characterization, and potential application in the preparation of chitosan–collagen biofilms. Bioresour. Technol. 2010, 101, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.C.G.; Marques, A.L.P.; Oliveira, S.M.; Diogo, G.S.; Pirraco, R.P.; Moreira-Silva, J.; Xavier, J.C.; Reis, R.L.; Silva, T.H.; Mano, J.F. Extraction and characterization of collagen from Antarctic and Sub Antarctic squid and its potential application in hybrid scaffolds for tissue engineering. Mater. Sci. Eng. C 2017, 78, 787–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-R.; Shiau, C.-Y.; Chen, H.-H.; Huang, B.-C. Isolation and characterization of acid and pepsin-solubilized collagens from the skin of balloon fish (Diodon holocanthus). Food Hydrocoll. 2011, 25, 1507–1513. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef]
- Schmidt, M.M.; Dornelles, R.C.P.; Mello, R.O.; Kubota, E.H.; Mazutti, M.A.; Kempka, A.P.; Demiate, I.M. Collagen extraction process. Int. Food Res. J. 2016, 23, 913–922. [Google Scholar]
- Mokhtar, N.D.; Wahab, W.A.; Hamdan, N.A.; Hadi, H.A.; Abu Hassan, M.S.; Bunnori, N.M. Extraction, optimization and characterization of collagen from chicken (Gallus gallus domesticus) feet. In Proceedings of the 5th International Conference on Chemical, Agricultural, Biological and Environmental Sciences, Kyoto, Japan, 18–19 April 2017; ISBN 978-81-933894-1-6. [Google Scholar]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiota. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Azzopardi, E.A.; Azzopardi, S.M.; Boyce, D.E.; Dickson, W.A. Emerging Gram-negative infections in burn wounds. J. Burn Care Res. 2011, 32, 570–576. [Google Scholar] [CrossRef]
- Khatami, F.; Robati, R.M.; Torabi-Rahvar, M.; Niu, W. Skin substitutes: Current applications and challenges. Front. Biomater. 2017, 5, 112–130. [Google Scholar]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A.; et al. Autologous bilayered self-assembled skin substitutes (SASSS) as permanent grafts: A case series of 14 severely burned patients indicating clinical effectiveness. Eur. Cells Mater. 2018, 36, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Ovaska, M.; Bertalan, Z.; Miksic, A.; Sugni, M.; Di Benedetto, C.; Ferrario, C.; Leggio, L.; Guidetti, L.; Alava, M.J.; La Porta, C.A.M.; et al. Deformation and fracture of echinoderm collagen networks. J. Mech. Behav. Biomed. Mater. 2017, 65, 42–52. [Google Scholar] [CrossRef]
- Faraj, K.A.; Van Kuppevelt, T.H.; Daamen, W.F. Construction of collagen scaffolds that mimic the three-dimensional architecture of specific tissues. Tissue Eng. 2007, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermueller, C.; Schwarz, S.; Elsaesser, A.F.; Sewing, J.; Baur, N.; van Bomhard, A.; Scheithauer, M.; Notbohm, H.; Rotter, N. Marine collagen scaffolds for nasal cartilage repair: Prevention of nasal septal perforations in a new orthotopic rat model using tissue engineering techniques. Tissue Eng. Part A 2013, 19, 2201–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, C.B.; Cukierman, E.; Artym, V.V. 3D Extracellular matrix from sectioned human tissues. Curr. Protoc. Cell. Biol. 2015, 62, 1620. [Google Scholar]
- Harley, B.A.C.; Gibson, L.J. In vivo and in vitro applications of collagen GAG scaffolds. Chem. Eng. J. 2008, 137, 102–121. [Google Scholar] [CrossRef]
- Elango, J.; Zhang, J.; Bao, B.; Palaniyandi, K.; Wang, S.; Wu, W.; Robinson, J.S. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef]
- Asanbe, O.; Hooper, R.; Elshazly, T.; Osoria, H.; Jacoby, A.; Joyce, J.; Weinreb, R.; Stroock, A.; Spector, J.; Walters, R.; et al. Innovative 3D collagen microsphere scaffold (MSS) promotes robust cellular invasion. Plast. Surg. 2014, 134, 28. [Google Scholar] [CrossRef]
- Sionkowska, A.; Slawomir, S.; Smiechowski, K.; Kolodziejczak, A. The review of versatile application of collagen. Polym. Adv. Technol. 2016, 28, 4–9. [Google Scholar] [CrossRef]
- Nguyen, B.B.; Moriarty, R.A.; Kamalitdinov, T.; Etheridge, J.M.; Fisher, J.P. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J. Biomed. Maters. Res. Part A 2017, 105A, 1123–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikholeslam, M.; Wright, M.E.E.; Jeschke, M.G.; Amini-Nik, S. Biomaterials for skin substitutes. Adv. Healthc. Mater. 2018, 7, 1700897. [Google Scholar] [CrossRef] [PubMed]







| E. coli | P. aeruginosa | S. aureus | |
|---|---|---|---|
| 1st experiment | 0 | 12200 | 2250 |
| 2nd experiment | 130 | 0 | 100 |
| 3rd experiment | 0 | 46400 | 0 |
| 4th experiment | 20 | 0 | 0 |
| 5th experiment | 0 | 0 | / |
| Mean | 30 | 11720 | 587.5 |
| % of infiltrated bacteria | 0.00030 | 0.11720 | 0.005875 |
| St. dev (+/−) | 0.0005 | 0.179 | 0.0096 |
| % of retained bacteria | 99.9997 | 99.8828 | 99.9941 |
| Animal Source | Collagen Extraction Yield (%) | Reference |
|---|---|---|
| Paracentrotus lividus (I) | 4.93 | Present study |
| Rhizostoma pulmo (I) | 0.2–1 | [35] |
| Chrysaora spp. (I) | 9–19 | [36] |
| Different squid species (I) | 1–11 | [37,38] |
| Diodon holocanthus (V) | 4–19 | [39] |
| Thunnus albacares (V) | 1.07–12.1 | [40] |
| Bovines, pigs, sheep (V) | 10–30 | [41] |
| Poultry (V) | 12.63–30.04 | [42] |
| Salmo salar (V) | 19.6 | [19] |
| Gadus morhua (V) | 10.9 | [19] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Mar. Drugs 2020, 18, 414. https://0-doi-org.brum.beds.ac.uk/10.3390/md18080414
Ferrario C, Rusconi F, Pulaj A, Macchi R, Landini P, Paroni M, Colombo G, Martinello T, Melotti L, Gomiero C, et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Marine Drugs. 2020; 18(8):414. https://0-doi-org.brum.beds.ac.uk/10.3390/md18080414
Chicago/Turabian StyleFerrario, Cinzia, Francesco Rusconi, Albana Pulaj, Raffaella Macchi, Paolo Landini, Moira Paroni, Graziano Colombo, Tiziana Martinello, Luca Melotti, Chiara Gomiero, and et al. 2020. "From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine" Marine Drugs 18, no. 8: 414. https://0-doi-org.brum.beds.ac.uk/10.3390/md18080414