Disparities in Temporal and Geographic Patterns of Myocardial Infarction Hospitalization Risks in Florida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Data Sources and Data Preparation
2.2.1. Hospital Discharge Data
2.2.2. Population Data
2.2.3. Cartographic Boundary Files
2.3. Descriptive Statistics
2.4. Identification of Geographic Clusters
2.5. Mapping of Hospitalization Risks and Clusters
2.6. Temporal Trends
2.7. Ethical Consideration
2.8. Data Availability
3. Results
3.1. Descriptive Analyses of MI Hospitalization
3.2. Spatial Patterns
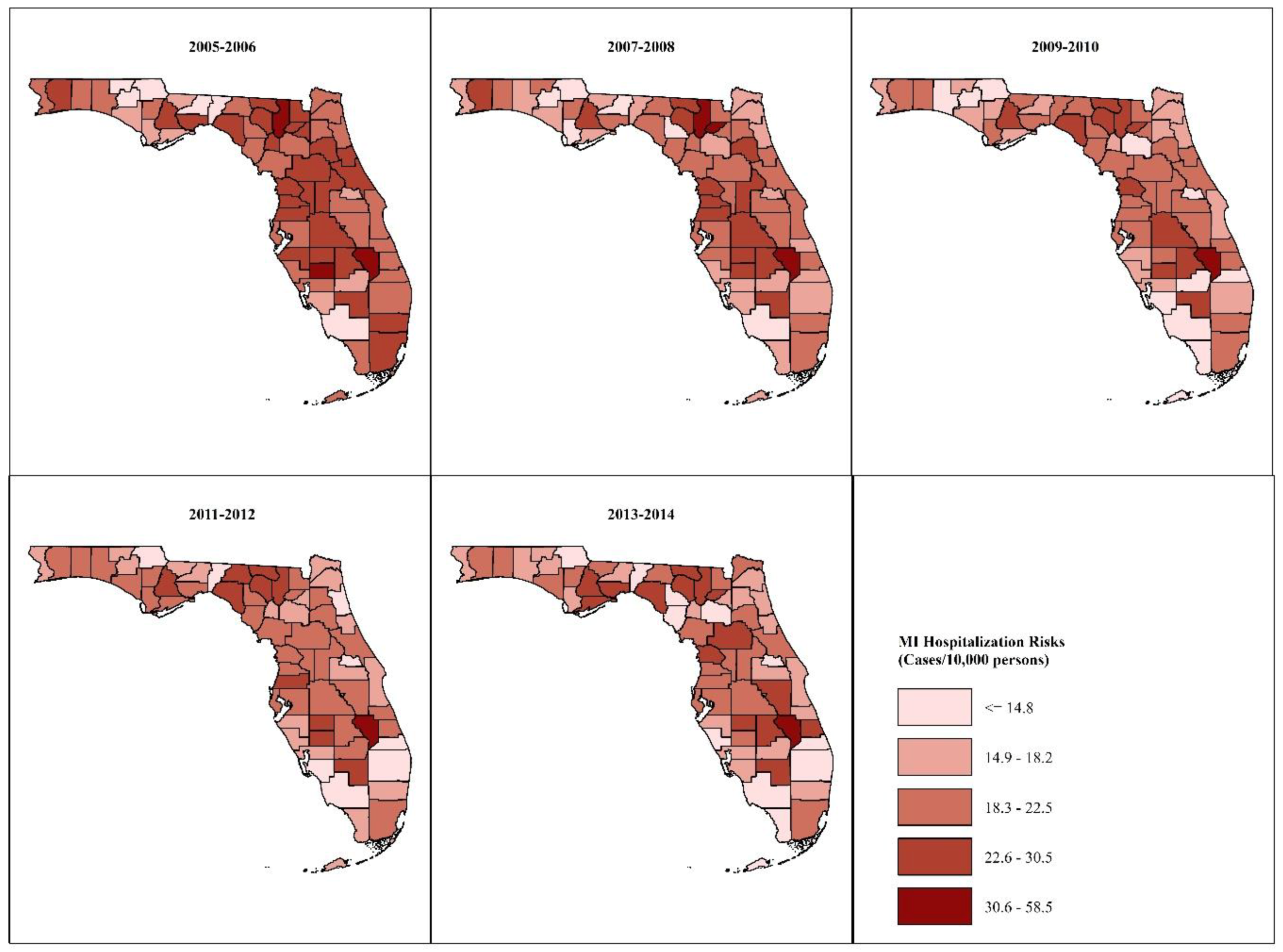
3.2.1. Age- and Sex-Adjusted MI Risks
3.2.2. Kulldorff’s Circular Spatial Scan Statistics (CSSS) Clusters
3.2.3. Tango’s Flexible Spatial Scan Statistics (FSSS) Clusters
- The identification of two high-risk circular and irregularly-shaped clusters in the Panhandle during the 2013–2014 time period. Kulldorff’s method identified a low-risk cluster in those counties during the 2005–2006 period.
- The identification of two distinct high-risk FSSS clusters in the Big Bend area as well as persistent clustering oh high-risks in DeSoto, Hardee, Highlands, Polk and Okeechobee counties in South Central Florida throughout the 10-year study period. In contrast, Kulldorff’s method identified one large cluster in the Big Bend area in three out of five of the 2-year time intervals assessed, and persistent clustering of high risks in Polk and Okeechobee counties only.
- The FSSS high-risk clusters only included counties with elevated risks. Kulldorff’s clusters, on the other hand, still included a few counties with elevated risks in low-risk clusters and counties with unelevated risks in high-risk clusters, despite using a window with a maximum size of 13.4% of the population in Florida. For instance, Hendry County was a part of the persistent low-risk cluster in southeast Florida despite having elevated relative risks ranging from 1.1–1.7 during the study period. Likewise, Sumter County was a constituent of the persistent high-risk cluster in the Big Bend area despite having unelevated relative risks of between 0.98–1.0 during the study period.
3.3. Temporal Trends
3.4. Health Disparities
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fang, J.; Alderman, M.H.; Keenan, N.L.; Ayala, C. Acute myocardial infarction hospitalization in the United States, 1979 to 2005. Am. J. Med. 2010, 123, 259–266. [Google Scholar] [CrossRef]
- Reynolds, K.; Go, A.S.; Leong, T.K.; Boudreau, D.M.; Cassidy-Bushrow, A.E.; Fortmann, S.P.; Goldberg, R.J.; Gurwitz, J.H.; Magid, D.J.; Margolis, K.L.; et al. Trends in Incidence of Hospitalized Acute Myocardial Infarction in the Cardiovascular Research Network (CVRN). Am. J. Med. 2017, 130, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Executive summary: Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, 399–410. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.W.; Elixhauser, A.; Sun, R. Trends in Hospital Inpatient Trends in Hospital Inpatient Stays in the United States, 2005–2014. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; HCUP Statistical Brief #225; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017. [Google Scholar]
- National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health; Services UDoHaH: Hyattsville, MD, USA, 2017.
- Ford, E.S. Trends in predicted 10-year risk of coronary heart disease and cardiovascular disease among U.S. adults from 1999 to 2010. J. Am. Coll. Cardiol. 2013, 61, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.W.; Sidney, S.; Chandra, M.; Sorel, M.; Selby, J.V.; Go, A.S. Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med. 2010, 362, 2155–2165. [Google Scholar] [CrossRef]
- Huffman, M.D.; Capewell, S.; Ning, H.; Shay, C.M.; Ford, E.S.; Lloyd-Jones, D.M. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: Results from the National Health and Nutrition Examination Surveys. Circulation 2012, 125, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Talbott, E.O.; Rager, J.R.; Brink, L.L.; Benson, S.M.; Bilonick, R.A.; Wu, W.C.; Han, Y.-Y. Trends in Acute Myocardial Infarction Hospitalization Rates for US States in the CDC Tracking Network. PLoS ONE 2013, 8, e64457. [Google Scholar] [CrossRef]
- Casper, M.; Kramer, M.R.; Quick, H.; Schieb, L.J.; Vaughan, A.S.; Greer, S. Changes in the Geographic Patterns of Heart Disease Mortality in the United States: 1973 to 2010. Circulation 2016, 133, 1171–1180. [Google Scholar] [CrossRef]
- Odoi, E.W.; Nagle, N.; Roberson, S.; Kintziger, K.W. Geographic disparities and temporal changes in risk of death from myocardial infarction in Florida, 2000–2014. BMC Public Health 2019, 19, 505. [Google Scholar] [CrossRef]
- Pickle, L.W.; Gillum, R.F. Geographic variation in cardiovascular disease mortality in US blacks and whites. J. Natl. Med. Assoc. 1999, 91, 545–556. [Google Scholar]
- Roberson, S.; Dutton, M.; Macdonald, M.; Odoi, A. Does Place of Residence or Time of Year Affect the Risk of Stroke Hospitalization and Death? A Descriptive Spatial and Temporal Epidemiologic Study. PLoS ONE 2016, 11, e0145224. [Google Scholar] [CrossRef] [PubMed]
- Schieb, L.J.; Mobley, L.R.; George, M.; Casper, M. Tracking stroke hospitalization clusters over time and associations with county-level socioeconomic and healthcare characteristics. Stroke 2013, 44, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Gillespie, K.N.; McBride, T.M. Diabetes burden and access to preventive care in the rural United States. J. Rural Health 2010, 26, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Meit, M.; Knudson, A.; Gilbert, T.; Yu, A.T.; Tanenbaum, E.; Ormson, E.; TenBroeck, S.; Bayne, A.; Popat, S. The 2014 Update of the Rural-Urban. Chartbook; Rural Health Reform Policy Research Center, University of North Dakota Center for Rural Health: Grand Forks, ND, USA, 2014. [Google Scholar]
- Wang, Y.; Beydoun, M.A. The obesity epidemic in the United States—Gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol. Rev. 2007, 29, 6–28. [Google Scholar] [CrossRef]
- American Lung Association. State of Tobacco Control: Florida. Available online: https://www.lung.org/our-initiatives/tobacco/reports-resources/sotc/state-grades/?setstate=FL#hide-tab-1 (accessed on 22 April 2019).
- Koren, A.; Steinberg, D.M.; Drory, Y.; Gerber, Y. Socioeconomic environment and recurrent coronary events after initial myocardial infarction. Ann. Epidemiol. 2012, 22, 541–546. [Google Scholar] [CrossRef]
- York, N.L.; Rayens, M.K.; Zhang, M.; Jones, L.G.; Casey, B.R.; Hahn, E.J. Strength of tobacco control in rural communities. J. Rural Health 2010, 26, 120–128. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. HDS-2: Reduce Coronary Heart Disease Deaths. Healthy People 2020. Available online: https://www.healthypeople.gov/ (accessed on 17 April 2018).
- Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Kutz, M.J.; Huynh, C.; Barber, R.M.; Shackelford, K.A.; Mackenbach, J.P.; van Lenthe, F.J.; et al. US County-Level Trends in Mortality Rates for Major Causes of Death, 1980–2014. JAMA 2016, 316, 2385–2401. [Google Scholar] [CrossRef]
- Smith, S.M.; McAuliffe, K.; Hall, J.M.; McDonough, C.W.; Gurka, M.J.; Robinson, T.O.; Sacco, R.L.; Pepine, C.; Shenkman, E.; Cooper-DeHoff, R.M. Hypertension in Florida: Data From the One Florida Clinical Data Research Network. Prev. Chronic Dis. 2018, 15, E27. [Google Scholar] [CrossRef]
- Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Mackenbach, J.P.; van Lenthe, F.J.; Mokdad, A.H.; Murray, C.J.L. Inequalities in Life Expectancy Among US Counties, 1980 to 2014: Temporal Trends and Key Drivers. JAMA Intern. Med. 2017, 177, 1003–1011. [Google Scholar] [CrossRef]
- Ezzati, M.; Friedman, A.B.; Kulkarni, S.C.; Murray, C.J. The reversal of fortunes: Trends in county mortality and cross-county mortality disparities in the United States. PLoS Med. 2008, 5, e66. [Google Scholar] [CrossRef]
- Florida Population Estimates. Available online: http://www.flhealthcharts.com/FLQUERY/Population/PopulationRpt.aspx (accessed on 13 January 2018).
- Klein, R.J.; Schoenborn, C.A. Age Adjustment Using the 2000 Projected U.S. Population; Healthy People 2010 Stat Notes, No. 20; National Center for Health Statistics: Hyattsville, MD, USA, 2001; pp. 1–10.
- Unite States Census Bureau. American FactFinder. Available online: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_SF1_QTP1&prodType=table (accessed on 20 January 2018).
- Li, C.; Ford, E.S.; Zhao, G.; Wen, X.J.; Gotway, C.A. Age adjustment of diabetes prevalence: Use of 2010 U.S. Census data. J. Diabetes 2014, 6, 451–461. [Google Scholar] [CrossRef] [PubMed]
- US Census Bureau. Cartographic Boundary Shapefiles-Counties. Available online: https://www.census.gov/geographies/mapping-files/time-series/geo/carto-boundary-file.2010.html (accessed on 7 January 2018).
- Kulldorff, M. SaTScan User Guide for version 9.4. 2015. Available online: https://www.satscan.org/cgi-bin/satscan/register.pl/SaTScan_Users_Guide.pdf?todo=process_userguide_download (accessed on 13 March 2018).
- Takahashi, K.; Yokoyama, T.; Tango, T. FleXScan v3.1: Software for the Flexi-ble Scan Statistic; National Institute of Public Health: Tokyo, Japan, 2013. [Google Scholar]
- Tango, T. A spatial scan statistic with a restricted likelihood ratio. Jpn. J. Biom. 2008, 29, 75–95. [Google Scholar] [CrossRef]
- Takahashi, K.; Kulldorff, M.; Tango, T.; Yih, K. A flexibly shaped space-time scan statistic for disease outbreak detection and monitoring. Int. J. Health Geogr. 2008, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- ESRI. ArcGIS Desktop: Release 10.6.1 Redlands; Environmental Systems Research Institute: Redlands, CA, USA, 2018. [Google Scholar]
- Prates, M.O.; Kulldorff, M.; Assuncao, R.M. Relative risk estimates from spatial and space-time scan statistics: Are they biased? Stat. Med. 2014, 33, 2634–2644. [Google Scholar] [CrossRef] [PubMed]
- Florida Department of Health Office of Rural Health. Rural County List. Available online: http://www.floridahealth.gov/programs-and-services/community-health/rural-health/_documents/rual-counties-2000-2010.pdf (accessed on 24 January 2018).
- Rosvall, M.; Chaix, B.; Lynch, J.; Lindstrom, M.; Merlo, J. The association between socioeconomic position, use of revascularization procedures and five-year survival after recovery from acute myocardial infarction. BMC Public Health 2008, 8, 44. [Google Scholar] [CrossRef]
- Chen, J.; Normand, S.L.; Wang, Y.; Drye, E.E.; Schreiner, G.C.; Krumholz, H.M. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee-for-service beneficiaries: Progress and continuing challenges. Circulation 2010, 121, 1322–1328. [Google Scholar] [CrossRef]
- Roger, V.L.; Weston, S.A.; Gerber, Y.; Killian, J.M.; Dunlay, S.M.; Jaffe, A.S.; Bell, M.R.; Kors, J.; Yawn, B.P.; Jacobsen, S.J. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation 2010, 121, 863–869. [Google Scholar] [CrossRef]
- Nallamothu, B.K.; Young, J.; Gurm, H.S.; Pickens, G.; Safavi, K. Recent trends in hospital utilization for acute myocardial infarction and coronary revascularization in the United States. Am. J. Cardiol. 2007, 99, 749–753. [Google Scholar] [CrossRef]
- Singh, J.A.; Lu, X.; Ibrahim, S.; Cram, P. Trends in and disparities for acute myocardial infarction: An analysis of Medicare claims data from 1992 to 2010. BMC Med. 2014, 12, 190. [Google Scholar] [CrossRef]
- Rosamond, W.D.; Chambless, L.E.; Heiss, G.; Mosley, T.H.; Coresh, J.; Whitsel, E.; Wagenknecht, L.; Ni, H.; Folsom, A.R. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987–2008. Circulation 2012, 125, 1848–1857. [Google Scholar] [CrossRef]
- Wang, O.J.; Wang, Y.; Chen, J.; Krumholz, H.M. Recent trends in hospitalization for acute myocardial infarction. Am. J. Cardiol. 2012, 109, 1589–1593. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Tisminetzky, M.; Tran, H.V.; Yarzebski, J.; Lessard, D.; Gore, J.M. Decade Long Trends (2001–2011) in the Incidence Rates of Initial Acute Myocardial Infarction. Am. J. Cardiol. 2019, 123, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.S.; Evans, J.C.; Larson, M.G.; Kannel, W.B.; Levy, D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: The Framingham Heart Study. Circulation 2004, 110, 522–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, H.; Coady, S.; Rosamond, W.; Folsom, A.R.; Chambless, L.; Russell, S.D.; Sorlie, P.D. Trends from 1987 to 2004 in sudden death due to coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J. 2009, 157, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Kontos, M.C.; Fritz, L.M.; Anderson, F.P.; Tatum, J.L.; Ornato, J.P.; Jesse, R.L. Impact of the troponin standard on the prevalence of acute myocardial infarction. Am. Heart J. 2003, 146, 446–452. [Google Scholar] [CrossRef]
- Ali, M.K.; Bullard, K.M.; Saaddine, J.B.; Cowie, C.C.; Imperatore, G.; Gregg, E.W. Achievement of goals in U.S. diabetes care, 1999–2010. N. Engl. J. Med. 2013, 368, 1613–1624. [Google Scholar] [CrossRef]
- Fornasini, M.; Yarzebski, J.; Chiriboga, D.; Lessard, D.; Spencer, F.A.; Aurigemma, P.; Gore, J.M.; Goldberg, R.J. Contemporary trends in evidence-based treatment for acute myocardial infarction. Am. J. Med. 2010, 123, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; He, D.; Zhang, W.; Walton, R.G. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J. Am. Coll. Cardiol. 2012, 60, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Muntner, P.; Levitan, E.B.; Brown, T.M.; Sharma, P.; Zhao, H.; Bittner, V.; Glasser, S.; Kilgore, M.; Yun, H.; Woolley, J.M.; et al. Trends in the prevalence, awareness, treatment and control of high low density lipoprotein-cholesterol among United States adults from 1999–2000 through 2009–2010. Am. J. Cardiol. 2013, 112, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Moran, A.E. Trends in the Prevalence, Awareness, Treatment, and Control of Hypertension Among Young Adults in the United States, 1999 to 2014. Hypertension 2017, 70, 736–742. [Google Scholar] [CrossRef]
- Forsyth, C.J.; Pathak, E.B.; Strom, J.A. De Facto regionalization of care for ST-elevation myocardial infarction in Florida, 2001–2009. Am. Heart J. 2012, 164, 681–688. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Biondi-Zoccai, G.; Marino, P. Transferring patients with ST-segment elevation myocardial infarction for mechanical reperfusion: A meta-regression analysis of randomized trials. Ann. Emerg Med. 2008, 52, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. BRFSS Prevalence & Trends Data. Available online: https://www.cdc.gov/brfss/brfssprevalence/ (accessed on 11 May 2018).
- Dwyer-Lindgren, L.; Freedman, G.; Engell, R.E.; Fleming, T.D.; Lim, S.S.; Murray, C.J.; Mokdad, A.H. Prevalence of physical activity and obesity in US counties, 2001–2011: A road map for action. Popul. Health Metr. 2013, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer-Lindgren, L.; Mokdad, A.H.; Srebotnjak, T.; Flaxman, A.D.; Hansen, G.M.; Murray, C.J. Cigarette smoking prevalence in US counties: 1996–2012. Popul. Health Metr. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, E.L.; Cruz-Cano, R.; Bernat, D.; Whitsel, L.; Huang, J.; Sherwin, C.; Robertson, R.M. Association between Florida’s smoke-free policy and acute myocardial infarction by race: A time series analysis, 2000–2013. Prev. Med. 2016, 92, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Deng, L.; Becker, D.J.; Bittner, V.; Levitan, E.B.; Rosenson, R.S.; Safford, M.M.; Muntner, P. Trends in mortality and recurrent coronary heart disease events after an acute myocardial infarction among Medicare beneficiaries, 2001–2009. Am. Heart J. 2015, 170, 249–255. [Google Scholar] [CrossRef]
- Chaudhry, S.I.; Khan, R.F.; Chen, J.; Dharmarajan, K.; Dodson, J.A.; Masoudi, F.A.; Wang, Y.; Krumholz, H.M. National trends in recurrent AMI hospitalizations 1 year after acute myocardial infarction in Medicare beneficiaries: 1999–2010. J. Am. Heart Assoc. 2014, 3, e001197. [Google Scholar] [CrossRef] [Green Version]
- Rose, K.M.; Suchindran, C.M.; Foraker, R.E.; Whitsel, E.A.; Rosamond, W.D.; Heiss, G.; Wood, J.L. Neighborhood disparities in incident hospitalized myocardial infarction in four U.S. communities: The ARIC surveillance study. Ann. Epidemiol. 2009, 19, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Ford, E.S.; Ajani, U.A.; Croft, J.B.; Critchley, J.A.; Labarthe, D.R.; Kottke, T.E.; Giles, W.H.; Capewell, S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N. Engl. J. Med. 2007, 356, 2388–2398. [Google Scholar] [CrossRef]
- Diez Roux, A.V. Residential environments and cardiovascular risk. J. Urban Health 2003, 80, 569–589. [Google Scholar] [CrossRef] [Green Version]
- Havranek, E.P.; Mujahid, M.S.; Barr, D.A.; Blair, I.V.; Cohen, M.S.; Cruz-Flores, S.; Davey-Smith, G.; Dennison-Himmelfarb, C.R.; Lauer, M.S.; Lockwood, D.W.; et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2015, 132, 873–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mensah, G.A.; Mokdad, A.H.; Ford, E.S.; Greenlund, K.J.; Croft, J.B. State of disparities in cardiovascular health in the United States. Circulation 2005, 111, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Cutler, J.; Desvigne-Nickens, P.; Fortmann, S.P.; Friedman, L.; Havlik, R.; Hogelin, G.; Marler, J.; McGovern, P.; Morosco, G.; et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: Findings of the national conference on cardiovascular disease prevention. Circulation 2000, 102, 3137–3147. [Google Scholar] [CrossRef] [Green Version]
- Pickle, L.W.; Mungiole, M.; Jones, G.K.; White, A.A. Atlas of United States Mortality; National Center for Health Statistics: Hyatttsville, MD, USA, 1996.
- Barker, L.E.; Kirtland, K.A.; Gregg, E.W.; Geiss, L.S.; Thompson, T.J. Geographic distribution of diagnosed diabetes in the U.S.: A diabetes belt. Am. J. Prev. Med. 2011, 40, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Naghavi, M.; Mokdad, A.H.; Murray, C.J.L. Trends and Patterns of Geographic Variation in Cardiovascular Mortality Among US Counties, 1980–2014. J. Am. Med. Assoc. 2017, 317, 1976–1992. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Siahpush, M. Widening socioeconomic inequalities in US life expectancy, 1980–2000. Int. J. Epidemiol. 2006, 35, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Douthit, N.; Kiv, S.; Dwolatzky, T.; Biswas, S. Exposing some important barriers to health care access in the rural USA. Public Health 2015, 129, 611–620. [Google Scholar] [CrossRef]
- Perrin, A. Digital Gap between Rural and Nonrural America Persists. Available online: http://pewrsr.ch/2qBuZY1 (accessed on 20 May 2018).
- Reschovsky, J.D.; Staiti, A.B. Access and quality: Does rural America lag behind? Health Aff. (Millwood) 2005, 24, 1128–1139. [Google Scholar] [CrossRef] [Green Version]
- Gunderson, A.; Menachemi, N.; Brummel-Smith, K.; Brooks, R. Physicians who treat the elderly in rural Florida: Trends indicating concerns regarding access to care. J. Rural Health 2006, 22, 224–228. [Google Scholar] [CrossRef]
- Aneja, S.; Ross, J.S.; Wang, Y.; Matsumoto, M.; Rodgers, G.P.; Bernheim, S.M.; Rathore, S.S.; Krumholz, H.M. US cardiologist workforce from 1995 to 2007: Modest growth, lasting geographic maldistribution especially in rural areas. Health Aff. (Millwood) 2011, 30, 2301–2309. [Google Scholar] [CrossRef]
- Pathak, E.B.; Forsyth, C.J.; Anic, G.; Tanner, J.P.; Comins, M.M.; Strom, J.A. Transfer travel times for primary percutaneous coronary intervention from low-volume and non-percutaneous coronary intervention-capable hospitals to high-volume centers in Florida. Ann. Emerg. Med. 2011, 58, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.I.; Story, M.T.; Nelson, M.C. Neighborhood environments: Disparities in access to healthy foods in the U.S. Am. J. Prev. Med. 2009, 36, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.D.; Roe, M.T.; Rumsfeld, J.S.; Shaw, R.E.; Brindis, R.G.; Fonarow, G.C.; Cannon, C.P. A call to ACTION (acute coronary treatment and intervention outcomes network): A national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ. Cardiovasc. Qual. Outcomes 2009, 2, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tynan, M.A.; Holmes, C.B.; Promoff, G.; Hallett, C.; Hopkins, M.; Frick, B. State and Local Comprehensive Smoke-Free Laws for Worksites, Restaurants, and Bars—United States, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Doogan, N.J.; Roberts, M.E.; Wewers, M.E.; Stanton, C.A.; Keith, D.R.; Gaalema, D.E.; Kurti, A.N.; Redner, R.; Cepeda-Benito, A.; Bunn, J.Y.; et al. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev. Med. 2017, 104, 79–85. [Google Scholar] [CrossRef]
- Spatz, E.S.; Beckman, A.L.; Wang, Y.; Desai, N.R.; Krumholz, H.M. Geographic Variation in Trends and Disparities in Acute Myocardial Infarction Hospitalization and Mortality by Income Levels, 1999–2013. JAMA Cardiol. 2016, 1, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Matthews, K.A.; Croft, J.B.; Liu, Y.; Lu, H.; Kanny, D.; Wheaton, A.G.; Cunningham, T.J.; Khan, L.K.; Caraballo, R.S.; Holt, J.B.; et al. Health-Related Behaviors by Urban-Rural County Classification—United States, 2013. MMWR Surveill. Summ. 2017, 66, 1–8. [Google Scholar] [CrossRef]
- Barber, S.; Hickson, D.A.; Wang, X.; Sims, M.; Nelson, C.; Diez-Roux, A.V. Neighborhood Disadvantage, Poor Social Conditions, and Cardiovascular Disease Incidence Among African American Adults in the Jackson Heart Study. Am. J. Public Health 2016, 106, 2219–2226. [Google Scholar] [CrossRef]
- Dupre, M.E.; George, L.K.; Liu, G.; Peterson, E.D. The cumulative effect of unemployment on risks for acute myocardial infarction. Arch. Intern. Med. 2012, 172, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Loucks, E.B.; Lynch, J.W.; Pilote, L.; Fuhrer, R.; Almeida, N.D.; Richard, H.; Agha, G.; Murabito, J.M.; Benjamin, E.J. Life-course socioeconomic position and incidence of coronary heart disease: The Framingham Offspring Study. Am. J. Epidemiol. 2009, 169, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Claeys, M.J.; Rajagopalan, S.; Nawrot, T.S.; Brook, R.D. Climate and environmental triggers of acute myocardial infarction. Eur. Heart J. 2017, 38, 955–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, D.; Qiao, Y.; Brown, N.E.; Wang, J. Racial and Ethnic Disparities in Influenza Vaccination among Adults with Chronic Medical Conditions Vary by Age in the United States. PLoS ONE 2017, 12, e0169679. [Google Scholar] [CrossRef]
- Namayande, M.S.; Nejadkoorki, F.; Namayande, S.M.; Dehghan, H. Spatial Hotspot Analysis of Acute Myocardial Infarction Events in an Urban Population: A Correlation Study of Health Problems and Industrial Installation. Iran. J. Public Health 2016, 45, 94–101. [Google Scholar] [PubMed]
- Olives, C.; Myerson, R.; Mokdad, A.H.; Murray, C.J.; Lim, S.S. Prevalence, awareness, treatment, and control of hypertension in United States counties, 2001–2009. PLoS ONE 2013, 8, e60308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.K. Florida Population Growth: Past Present and Future; Bureau of Economic and Business Research, University of Florida: Gainesville, FL, USA, 2005. [Google Scholar]
- Deming, W.G. A decade of economic change and population shifts in U.S. regions. Mon. Labor Rev. 1996, 119, 3–12. [Google Scholar]
- Gaalema, D.E.; Elliott, R.J.; Morford, Z.H.; Higgins, S.T.; Ades, P.A. Effect of Socioeconomic Status on Propensity to Change Risk Behaviors Following Myocardial Infarction: Implications for Healthy Lifestyle Medicine. Prog. Cardiovasc. Dis. 2017, 60, 159–168. [Google Scholar] [CrossRef]
- University of Wisconsin Population Health Institute. County Health Rankings & Roadmaps. Available online: http://www.countyhealthrankings.org/ (accessed on 24 April 2018).
- Hobbs, M.; Duncan, M.J.; Collins, P.; McKenna, J.; Schoeppe, S.; Rebar, A.L.; Alley, S.; Short, C.; Vandelanotte, C. Clusters of health behaviours in Queensland adults are associated with different socio-demographic characteristics. J. Public Health 2018, 41, 268–277. [Google Scholar] [CrossRef]
- White, K.; Stewart, J.E.; Lopez-DeFede, A.; Wilkerson, R.C. Small-area Variation in Hypertension Prevalence among Black and White Medicaid Enrollees. Ethn. Dis. 2016, 26, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Health Council of South Florida. Mobilizing for Action through Partnerships and Planning (MAPP) Miami 2013–2018 Community Health Priorities, Goals & Strategies. 2013. Available online: http://miamidade.floridahealth.gov/about-us/_documents/miami-dade-cha-2013-20181.pdf (accessed on 27 April 2018).
- DuBard, C.A.; Garrett, J.; Gizlice, Z. Effect of language on heart attack and stroke awareness among U.S. Hispanics. Am. J. Prev. Med. 2006, 30, 189–196. [Google Scholar] [CrossRef]
- Almufleh, A.; Gabriel, T.; Tokayer, L.; Comerford, M.; Alaqeel, A.; Kurlansky, P. Role of community health outreach program “living for health” in improving access to federally qualified health centers in Miami-dade county, Florida: A cross-sectional study. BMC Health Serv. Res. 2015, 15, 181. [Google Scholar] [CrossRef] [Green Version]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E., Jr.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, 529–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garfield, R.; Orgera, K.; Damico, A. The Coverage Gap: Uninsured Poor Adults in States that Do Not Expand Medicaid—An Update. Available online: http://kff.org/health-reform/issue-brief/the-coverage-gap-uninsured-poor-adults-in-states-that-do-not-expand-medicaid-an-update/ (accessed on 23 June 2019).
- Smolderen, K.G.; Spertus, J.A.; Nallamothu, B.K.; Krumholz, H.M.; Tang, F.; Ross, J.S.; Ting, H.H.; Alexander, K.P.; Rathore, S.S.; Chan, P.S. Health care insurance, financial concerns in accessing care, and delays to hospital presentation in acute myocardial infarction. JAMA 2010, 303, 1392–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Survey of EMS Practices for Heart Disease and Stroke Florida Summary of Results; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2011.
- Granger, C.B.; Henry, T.D.; Bates, W.E.; Cercek, B.; Weaver, W.D.; Williams, D.O. Development of systems of care for ST-elevation myocardial infarction patients: The primary percutaneous coronary intervention (ST-elevation myocardial infarction-receiving) hospital perspective. Circulation 2007, 116, e55–e59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwitz, J.R.; Nichols, A.; Nallamothu, B.K.; Sasson, C.; Iwashyna, T.J. Expansion of invasive cardiac services in the United States. Circulation 2013, 128, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Scharff, D.P.; Mathews, K.J.; Jackson, P.; Hoffsuemmer, J.; Martin, E.; Edwards, D. More than Tuskegee: Understanding mistrust about research participation. J. Health Care Poor Underserved 2010, 21, 879–897. [Google Scholar] [CrossRef]
- Goraya, T.Y.; Jacobsen, S.J.; Kottke, T.E.; Frye, R.L.; Weston, S.A.; Roger, V.L. Coronary heart disease death and sudden cardiac death: A 20-year population-based study. Am. J. Epidemiol. 2003, 157, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Mannsverk, J.; Wilsgaard, T.; Mathiesen, E.B.; Lochen, M.L.; Rasmussen, K.; Thelle, D.S.; Njolstad, I.; Hopstock, L.A.; Bonaa, K.H. Trends in Modifiable Risk Factors Are Associated With Declining Incidence of Hospitalized and Nonhospitalized Acute Coronary Heart Disease in a Population. Circulation 2016, 133, 74–81. [Google Scholar] [CrossRef]
- Patel, A.; Fang, J.; Gillespie, C.; Odom, E.; Luncheon, C.; Ayala, C. Awareness of Heart Attack Signs and Symptoms and Calling 9-1-1 Among U.S. Adults. J. Am. Coll. Cardiol. 2018, 71, 808–809. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Hearth Disease: Heart Attack Signs and Symptoms. Available online: https://www.cdc.gov/heartdisease/signs_symptoms.htm (accessed on 27 March 2018).
- Hand, M.; Brown, C.; Horan, M.; Simons-Morton, D. The National Heart Attack Alert Program: Progress at 5 Years in Educating Providers, Patients, and the Public and Future Directions. J. Thromb. Thrombolysis 1998, 6, 9–17. [Google Scholar] [CrossRef]
- American Heart Association. Heart Attack: Warning Signs of a Heart Attack. Available online: https://www.heart.org/en/health-topics/heart-attack/warning-signs-of-a-heart-attack (accessed on 27 March 2018).
- Li, Y.; Rukshin, I.; Pan, F.; Sen, S.; Islam, M.; Yousif, A.; Rukshin, V. The impact of the 2008–2009 economic recession on acute myocardial infarction occurrences in various socioeconomic areas of raritan bay region, New Jersey. N. Am. J. Med. Sci. 2014, 6, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Phelan, J.C.; Link, B.G. Controlling disease and creating disparities: A fundamental cause perspective. J. Gerontol. B Psychol. Sci. Soc. Sci. 2005, 60, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.W.; Normand, S.L.; Wang, Y.; Barr, C.D.; Dominici, F. Geographic disparities in the incidence and outcomes of hospitalized myocardial infarction: Does a rising tide lift all boats? Circ. Cardiovasc. Qual. Outcomes 2012, 5, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortman, J.M.; Velkoff, V.A.; Hogan, H. An Aging Nation: The Older Population in the United States, Current Population Reports; U.S. Census Bureau: Washington, DC, USA, 2014; pp. 25–1140.
- Williams, J.L. Projecting the general cardiology workforce shortage. Am. Heart Hosp. J. 2007, 5, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Tango, T.; Takahashi, K. A flexible spatial scan statistic with a restricted likelihood ratio for detecting disease clusters. Stat. Med. 2012, 31, 4207–4218. [Google Scholar] [CrossRef] [PubMed]
- Jacquez, G.M. Cluster Morphology Analysis. Spat. Spatio Temporal Epidemiol. 2009, 1, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Szklo, M.; Nieto, F.J. Epidemiology: Beyond the Basics, 4th ed.; Jones and Bartlett: Burlington, MA, USA, 2019; p. 565. [Google Scholar]




| Characteristic | % of Total Cases | Age-Adjusted Risks/10,000 Persons (95% CI) | % Change | ||
|---|---|---|---|---|---|
| 2005–2006 | 2013–2014 | 2005–2006 | 2013–2014 | ||
| Total MI cases | 92,261 | 84,172 | 22.0 | 17.7 | 19.5 |
| Sex | |||||
| Male | 60.0 | 61.7 | 29.7 (29.4, 29.9) | 23.9 (23.7, 24.1) | −19.6 |
| Female | 40.0 | 38.3 | 15.7 (15.6, 15.9) | 12.1 (12.0, 12.2) | −23.0 |
| c Age (years) | |||||
| 0–34 | 0.7 | 0.7 | 0.4 (0.4, 0.5) | 0.4 (0.3, 0.4) | −14.6 |
| 35–44 | 4.5 | 3.7 | 8.1 (7.8, 8.3) | 6.5 (6.2, 6.7) | −20.2 |
| 45–54 | 12.7 | 12.9 | 23.3 (22.9, 23.7) | 19.8 (19.4, 20.1) | −15.1 |
| 55–64 | 18.9 | 21.8 | 43.2 (42.5, 43.8) | 36.9 (36.4, 37.4) | −14.6 |
| 65–74 | 22.0 | 24.6 | 68.3 (67.4, 69.3) | 53.4 (52.7, 54.1) | −21.8 |
| 75–84 | 25.8 | 21.6 | 103.7 (102.3, 105.0) | 79.6 (78.4, 80.7) | −23.2 |
| ≥85 | 15.4 | 14.7 | 168.7 (165.9, 171.5) | 124.9 (122.7, 127.1) | −26.0 |
| z Race | |||||
| White | 87.7 | 80.9 | 21.1 (22.0, 22.3) | 16.5 (16.4, 16.6) | −25.5 |
| Black | 8.5 | 10.7 | 20.2 (19.7, 20.6) | 17.4 (17.0, 17.8) | −13.8 |
| All other races | 2.4 | 7.2 | 21.7 (20.8, 22.6) | 41.4 (403, 42.4) | +90.4 |
| β Ethnicity | |||||
| Hispanic | 12.1 | 15.3 | 19.5 (19.2, 19.9) | 16.8 (16.5, 17.1) | −14.1 |
| Non-Hispanic | 86.6 | 82.2 | 22.4 (22.2, 22.6) | 17.4 (17.3, 17.5) | −22.4 |
| Rural/Urban | |||||
| Rural | 5.6 | 6.8 | 24.7 (24.0, 25.4) | 21.9 (21.3, 22.5) | −11.5 |
| Urban | 94.4 | 93.2 | 22.1 (21.9, 22.2) | 17.4 (17.3, 17.5) | −21.3 |
| Characteristic | % of Total Cases | Age-Adjusted Risks/10,000 Persons (95% CI) | % Change | ||
|---|---|---|---|---|---|
| 2005–2006 | 2013–2014 | 2005–2006 | 2013–2014 | ||
| Total MI cases | 92,261 | 84,172 | 20.0 | 16.0 | 20 |
| Sex | |||||
| Male | 60.0 | 61.7 | 26.9 (26.6, 27.1) | 21.5 (21.3, 21.66) | −20.0 |
| Female | 40.0 | 38.3 | 14.4 (14.3, 14.6) | 11.1 (10.9, 11.2) | −23.3 |
| c Age (years) | |||||
| 0–34 | 0.7 | 0.7 | 0.4 (0.4, 0. 5) | 0.4 (0.3, 0.4) | −14.6 |
| 35–44 | 4.5 | 3.7 | 8.1 (7.8, 8.3) | 6.5 (6.2, 6.7) | −20.2 |
| 45–54 | 12.7 | 12.9 | 23.3 (22.9, 23.7) | 19.8 (19.4, 20.1) | −15.1 |
| 55–64 | 18.9 | 21.8 | 43.2 (42.5, 43.8) | 36.9 (36.4, 37.4) | −14.6 |
| 65–74 | 22.0 | 24.6 | 68.3 (67.4, 69.3) | 53.4 (52.7, 54.1) | −21.8 |
| 75–84 | 25.8 | 21.6 | 103.7 (102.3, 105.0) | 79.6 (78.4, 80.7) | −23.2 |
| ≥85 | 15.4 | 14.7 | 168.7 (165.9, 171.5) | 124.9 (122.7, 127.1) | −26.0 |
| z Race | |||||
| White | 87.7 | 80.9 | 20.1 (20.0, 20.3) | 14.9 (14.8, 15.0) | −25.9 |
| Black | 8.5 | 10.7 | 18.3 (17.9, 18.8) | 15.8 (15.5, 16.1) | −13.8 |
| All other races | 2.4 | 7.2 | 19.8 (19.0, 20.6) | 37.6 (36.7, 38.6) | +89.9 |
| β Ethnicity | |||||
| Hispanic | 12.1 | 15.3 | 17.7 (17.4, 18.1) | 15.2 (14.9, 15.4) | −14.4 |
| Non-Hispanic | 86.6 | 82.2 | 20.4 (20.3, 20.6) | 15.8 (15.7, 15.9) | −22.6 |
| Rural/Urban | |||||
| Rural | 5.6 | 6.8 | 22.4 (21.8, 23.0) | 19.8 (19.2, 20.3) | −11.8 |
| Urban | 94.4 | 93.2 | 20.1 (20.0, 20.2) | 15.7 (15.6, 15.9) | −21.7 |
| Time Interval | Cluster | County | Cluster Population (% of Florida Population) | Observed # of Hospitalizations | Expected # of Hospitalizations | # of Cases/10,000 Persons | p-Value |
|---|---|---|---|---|---|---|---|
| 2005–2006 | 1 | 86 | 4,828,792 (13.4) | 11,961 | 10,467.24 | 29.5 | <0.00001 |
| 2 | 75, 17, 41, 29, 53, 1, 83, 67, 119, 101, 121, 125, 123, 7, 23, 69, 107 | 3,883,180 (10.8) | 13,360 | 11,928.25 | 28.5 | <0.00001 | |
| 3 | 49, 27, 55, 105 | 1,427,730 (4.0) | 4843 | 3979.55 | 31.1 | <0.00001 | |
| 4 | 93 | 77,985 (0.2) | 319 | 190.53 | 42.8 | <0.00001 | |
| 5 | 81 | 620,203 (1.7) | 2214 | 1984.07 | 28.5 | 0.00013 | |
| 2007–2008 | 1 | 75, 17, 41, 29, 53, 1, 83, 67, 119, 101, 121, 125, 123, 7, 23, 69, 107 | 4,093,374 (11.0) | 13,047 | 11,445.77 | 26.6 | <0.00001 |
| 2 | 86 | 4,931,242 (13.3) | 11,042 | 9887.13 | 26.1 | <0.00001 | |
| 3 | 55, 93 | 278,730 (0.8) | 1266 | 902.22 | 32.7 | <0.00001 | |
| 4 | 103, 81, 57 | 4,894,293 (13.2) | 12,315 | 11,451.80 | 25.1 | <0.00001 | |
| 5 | 127 | 997,928 (2.7) | 3009 | 2729.53 | 25.7 | <0.00001 | |
| 2009–2010 | 1 | 93 | 79,951 (0.2) | 392 | 167.89 | 51.4 | <0.00001 |
| 2 | 75, 17, 41, 29, 53, 1, 83, 67, 119, 101, 121, 125, 123, 7, 23, 69, 107 | 4,154,803 (11.1) | 12,149 | 10,791.06 | 24.8 | <0.00001 | |
| 3 | 105, 49, 97, 57 | 4,241,326 (11.3) | 9068 | 7893.89 | 25.3 | <0.00001 | |
| 4 | 86 | 4,982,221 (13.3) | 10,704 | 9501.80 | 24.8 | <0.00001 | |
| 2011–2012 | 1 | 93 | 79,765 (0.2) | 532 | 163.13 | 70.6 | <0.00001 |
| 2 | 17, 53, 75, 101, 119, 83, 69 | 3,116,403 (8.2) | 10,296 | 8600.95 | 25.9 | <0.00001 | |
| 3 | 105, 49, 97 | 1,828,195 (4.8) | 4359 | 3630.45 | 26.0 | <0.00001 | |
| 4 | 86 | 5,056,071 (13.3) | 10,495 | 9565.79 | 23.8 | <0.00001 | |
| 5 | 47, 121, 23, 79, 67, 3, 125 | 393,872 (1.0) | 1007 | 776.93 | 28.1 | <0.00001 | |
| 6 | 103 | 1,836,685 (4.8) | 5225 | 4764.21 | 23.6 | <0.00001 | |
| 7 | 91, 131, 113 | 790,131 (2.0) | 1730 | 1498.07 | 25.0 | <0.00001 | |
| 2013–2014 | 1 | 93 | 79,952 (0.2) | 532 | 162.99 | 70.7 | <0.00001 |
| 2 | 75, 17, 41, 29, 53, 1, 83, 67, 119, 101, 121, 125, 123, 7, 23, 69, 107 | 4,276,132 (11.0) | 13,006 | 10,812.73 | 25.7 | <0.00001 | |
| 3 | 105, 49, 97 | 1,887,107 (4.9) | 4645 | 3729.85 | 27.0 | <0.00001 | |
| 4 | 86 | 5,198,431 (13.4) | 10,440 | 9840.38 | 23.3 | <0.00001 | |
| 5 | 127 | 1,003,522 (2.6) | 2818 | 2528.09 | 24.2 | 0.00013 |
| Time Interval | Cluster | County | Cluster Population (% of Florida Population) | Observed # of Hospitalizations | Expected # of Hospitalizations | # of Cases/10,000 Persons | p-Value |
|---|---|---|---|---|---|---|---|
| 2005–2006 | 1 | 51, 43, 21, 71 | 1,853,327 (5.1) | 4290 | 5816.98 | 23.9 | <0.00001 |
| 2 | 63, 13, 133, 59, 39, 77, 5, 131, 45, 73 | 1,308,614 (3.6) | 1988 | 2690.58 | 18.7 | <0.00001 | |
| 3 | 117, 95 | 2,977,058 (8.2) | 4719 | 5259.91 | 22.9 | <0.00001 | |
| 4 | 85, 111, 99 | 3,353,826 (9.3) | 9590 | 10,269.14 | 23.9 | <0.00001 | |
| 5 | 31, 89, 19, 109 | 2,487,636 (6.9) | 4555 | 4987.02 | 23.4 | <0.00001 | |
| 2007–2008 | 1 | 71, 15, 51, 27, 21, 43, 115 | 3,085,443 (8.3) | 8078 | 9688.30 | 19.5 | <0.00001 |
| 2 | 63, 13, 133, 59, 39, 77, 5, 131, 45, 73 | 1,345,777 (3.6) | 1940 | 2582.27 | 17.5 | <0.00001 | |
| 3 | 9, 97, 95, 61, 117 | 4,954,523 (13.3) | 8728 | 9708.59 | 21.0 | <0.00001 | |
| 4 | 31, 89, 19, 109 | 2,580,453 (6.9) | 4141 | 4843.12 | 20.0 | <0.00001 | |
| 5 | 99, 85 | 2902376 (7.8) | 7241 | 8075.73 | 20.9 | <0.00001 | |
| 2009–2010 | 1 | 71, 15, 51, 27, 21, 43, 115 | 3,127,273 (8.3) | 7227 | 9173.95 | 17.4 | <0.00001 |
| 2 | 99, 85 | 2,926,434 (7.8) | 6304 | 7649.28 | 18.2 | <0.00001 | |
| 3 | 117 | 844,417 (2.2) | 1154 | 1492.38 | 17.0 | <0.00001 | |
| 4 | 133, 59, 5, 63, 131 | 636,666 (1.7) | 1029 | 1327.01 | 17.1 | <0.00001 | |
| 5 | 31, 89 | 1,870,728 (5.0) | 2890 | 3234.16 | 19.7 | <0.00001 | |
| 6 | 73 | 550,260 (1.5) | 668 | 805.64 | 18.3 | <0.00001 | |
| 2011–2012 | 1 | 99, 85 | 2,954,576 (7.8) | 5936 | 7615.18 | 16.9 | <0.00001 |
| 2 | 71, 15, 51, 27, 21, 43, 115 | 3,173,919 (8.4) | 7448 | 9106.19 | 17.7 | <0.00001 | |
| 3 | 117, 95 | 3,184,374 (8.4) | 4187 | 5050.44 | 18.0 | <0.00001 | |
| 4 | 109 | 391,071 (1.0) | 659 | 821.37 | 17.4 | <0.00001 | |
| 5 | 65, 73 | 582,855 (1.5) | 725 | 882.46 | 17.8 | 0.00002 | |
| 6 | 63 | 100,092 (0.3) | 145 | 205.78 | 15.3 | 0.0022 | |
| 2013–2014 | 1 | 99, 85 | 3,011,105 (7.7) | 5839 | 7661.46 | 16.9 | <0.00001 |
| 2 | 71, 15, 51, 27, 21, 43, 115 | 3,270,757 (8.4) | 7391 | 9290.84 | 16.5 | <0.00001 | |
| 3 | 117 | 868,598 (2.2) | 1163 | 1569.40 | 16.1 | <0.00001 | |
| 4 | 11 | 3,582,137 (9.2) | 6301 | 7146.82 | 19.1 | <0.00001 | |
| 5 | 63 | 100,080 (0.3) | 117 | 207.34 | 12.2 | <0.00001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odoi, E.W.; Nagle, N.; DuClos, C.; Kintziger, K.W. Disparities in Temporal and Geographic Patterns of Myocardial Infarction Hospitalization Risks in Florida. Int. J. Environ. Res. Public Health 2019, 16, 4734. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16234734
Odoi EW, Nagle N, DuClos C, Kintziger KW. Disparities in Temporal and Geographic Patterns of Myocardial Infarction Hospitalization Risks in Florida. International Journal of Environmental Research and Public Health. 2019; 16(23):4734. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16234734
Chicago/Turabian StyleOdoi, Evah W., Nicholas Nagle, Chris DuClos, and Kristina W. Kintziger. 2019. "Disparities in Temporal and Geographic Patterns of Myocardial Infarction Hospitalization Risks in Florida" International Journal of Environmental Research and Public Health 16, no. 23: 4734. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16234734





