Urinary Metabolites of Organophosphate Pesticides among Pregnant Women Participating in the Japan Environment and Children’s Study (JECS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Sample Collection
2.2. Chemicals and Reagents
2.3. Sample Preparation
2.4. Instrument Analysis and Calculations
2.5. QC
2.6. Data Collection
2.7. Data Analysis
2.8. Cumulative Risk Assessment
3. Results
3.1. Method Performance
3.2. Concentrations of DAPs in Maternal Urine Samples
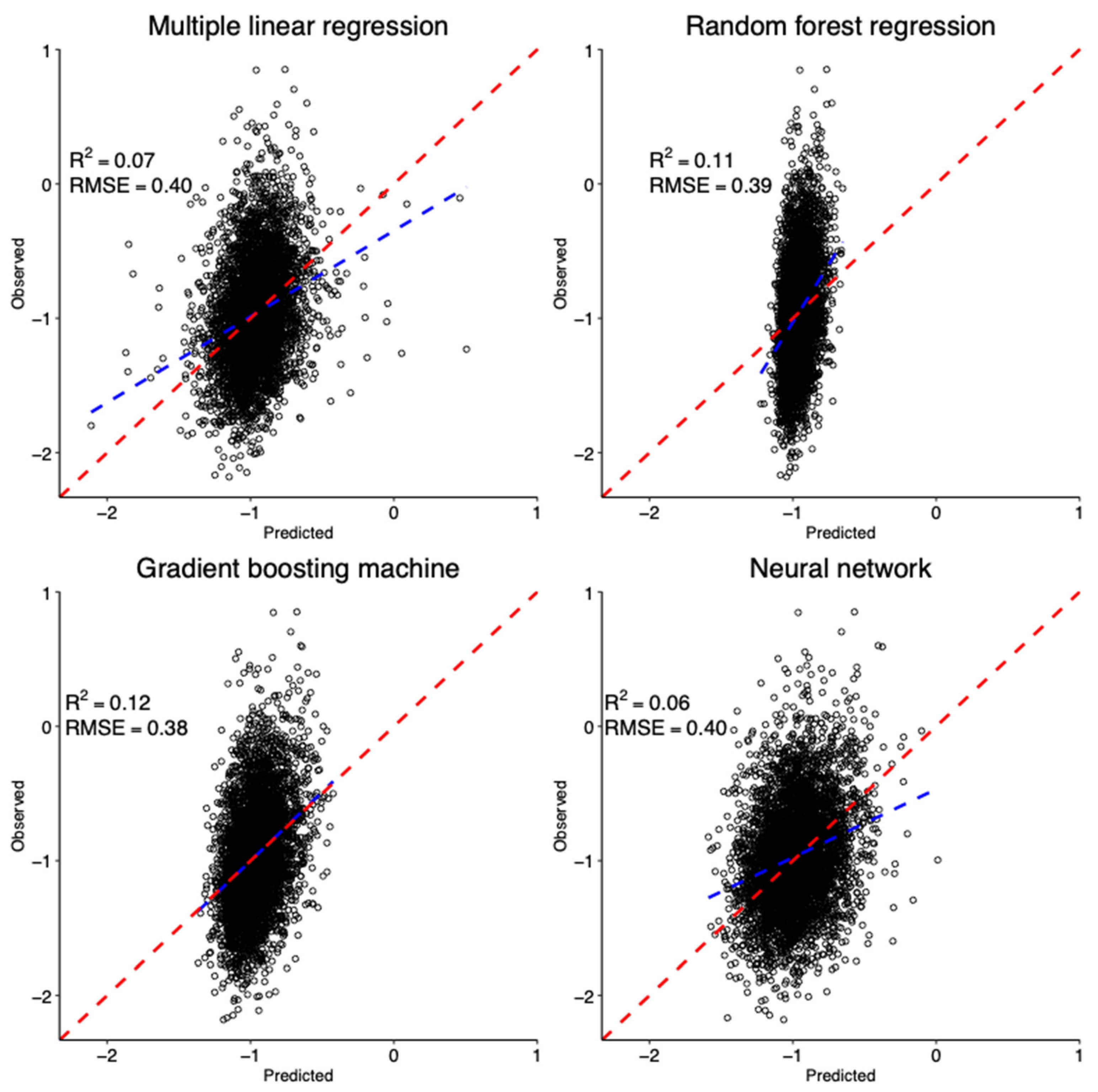
3.3. Predictors of DAPs in Maternal Urine Samples
3.4. Cumulative Risk Assessment
4. Discussion
4.1. Concentrations of DAPs in Maternal Urine Samples
4.2. Predictors of DAPs in Maternal Urine Samples
4.3. Cumulative Risk Assessment
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| BMD | benchmark dose |
| BMD10 | 10% onset of toxicity is used for general toxicity as the BMR |
| BMDL | BMD lower confidence limit |
| BMI | body mass index |
| BMR | bench-mark response |
| DAPs | dialkylphosphates |
| DEDTP | diethyldithiophosphate |
| DEP | diethylphosphate |
| DEs | diethyl DAP (DEP + DETP) |
| DETP | diethylthiophosphate |
| DMDTP | dimethyldithio-phosphate |
| DMP | dimethylphosphate |
| DMs | dimethyl DAP (DMP + DMTP + DMDTP) |
| DMTP | dimethylthiophosphate |
| DR | detection rate |
| G-EQUAS | German External Quality Assessment Scheme |
| GBM | gradient boosting machine |
| IQR | interquartile range |
| LC-MS/MS | liquid chromatography-tandem mass spectrometer |
| LCMRL | lowest concentration minimum reporting level |
| MICE | multiple imputation by chained equations |
| MOE | margin of exposure |
| MRL | minimum reporting level |
| MW | molecular weight |
| NOAEL | no observed adverse effect level |
| OPPs | organophosphate pesticides |
| QC | quality control |
| QRLIC | quantile regression approach for left-censored missing |
| RFPs | elative potency factors |
| RFR | random forest regression |
| RSD | relative standard deviation. |
| SD | standard deviation |
| U.S. EPA | The U.S. Environmental Protection Agency |
References
- Slotkin, T.A. Cholinergic systems in brain development and disruption by neurotoxicants: Nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G. Organophosphorus compounds at 80: Some old and new issues. Toxicol. Sci. 2018, 162, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Han, S.; Liang, D.; Shi, X.; Wang, F.; Liu, W.; Zhang, L.; Chen, L.; Gu, Y.; Tian, Y. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: A birth cohort study in Shenyang, China. PLoS ONE 2014, 9, e88491. [Google Scholar] [CrossRef] [PubMed]
- Cartier, C.; Warembourg, C.; Le Maner-Idrissi, G.; Lacroix, A.; Rouget, F.; Monfort, C.; Limon, G.; Durand, G.; Saint-Amour, D.; Cordier, S.; et al. Organophosphate insecticide metabolites in prenatal and childhood urine samples and intelligence scores at 6 years of age: Results from the mother-child PELAGIE cohort (France). Environ. Health Perspect. 2016, 124, 674–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Wu, C.; Chang, X.; Qi, X.; Zheng, M.; Zhou, Z. Adverse associations of both prenatal and postnatal exposure to organophosphorous pesticides with infant neurodevelopment in an agricultural area of Jiangsu Province, China. Environ. Health Perspect. 2016, 124, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Wang, C.; Hum, Y.; Gao, Y.; Zhou, Y.; Shi, R.; Zhang, Y.; Kamijima, M.; Ueyama, J.; et al. Association between organophosphate pesticide exposure and thyroid hormones in pregnant women. Epidemiology 2017, 28, S35–S40. [Google Scholar] [CrossRef]
- Ye, M.; Beach, J.; Martin, J.W.; Senthilselvan, A. Urinary dialkyl phosphate concentrations and lung function parameters in adolescents and adults: Results from the Canadian Health Measures Survey. Environ. Health Perspect. 2016, 124, 491–497. [Google Scholar] [CrossRef] [Green Version]
- González-Alzaga, B.; Romero-Molina, D.; López-Flores, I.; Giménez-Asensio, M.J.; Hernández, A.F.; Lacasaña, M. Urinary levels of organophosphate pesticides and predictors of exposure in pre-school and school children living in agricultural and urban communities from south Spain. Environ. Res. 2020, 186, 109459. [Google Scholar] [CrossRef]
- Suwannarin, N.; Prapamontol, T.; Isobe, T.; Nishihama, Y.; Nakayama, S.F. Characteristics of exposure of reproductive-age farmworkers in Chiang Mai Province, Thailand, to organophosphate and neonicotinoid insecticides: A pilot study. Int. J. Environ. Res. Public Health 2020, 17, 7871. [Google Scholar] [CrossRef]
- Sokoloff, K.; Fraser, W.; Arbuckle, T.E.; Fisher, M.; Gaudreau, E.; LeBlanc, A.; Morisset, A.S.; Bouchard, M.F. Determinants of urinary concentrations of dialkyl phosphates among pregnant women in Canada—Results from the MIREC study. Environ. Int. 2016, 94, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Dries, M.A.; Pronk, A.; Guxens, M.; Spaan, S.; Voortman, T.; Jaddoe, V.W.; Jusko, T.A.; Longnecker, M.P.; Tiemeier, H. Determinants of organophosphate pesticide exposure in pregnant women: A population-based cohort study in the Netherlands. Int. J. Hyg. Environ. Health 2018, 221, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Ministry of the Environment. The Estimated Releases Outside Notification (2011–2014). Available online: https://www.env.go.jp/chemi/prtr/result/todokedegai_siryo.html (accessed on 23 December 2020).
- Suárez-Jacobo, A.; Alcantar-Rosales, V.M.; Alonso-Segura, D.; Heras-Ramírez, M.; Elizarragaz-De La Rosa, D.; Lugo-Melchor, O.; Gaspar-Ramirez, O. Pesticide residues in orange fruit from citrus orchards in Nuevo Leon State, Mexico. Food Addit. Contam. Part B Surveill. 2017, 10, 192–199. [Google Scholar] [CrossRef]
- Ueyama, J.; Harada, K.H.; Koizumi, A.; Sugiura, Y.; Kondo, T.; Saito, I.; Kamijima, M. Temporal levels of urinary neonicotinoid and dialkylphosphate concentrations in Japanese women between 1994 and 2011. Environ. Sci. Technol. 2015, 49, 14522–14528. [Google Scholar] [CrossRef]
- Li, A.J.; Kannan, K. Profiles of urinary neonicotinoids and dialkylphosphates in populations in nine countries. Environ. Int. 2020, 145, 106120. [Google Scholar] [CrossRef]
- Fenske, R.A.; Lu, C.; Simcox, N.J.; Loewenherz, C.; Touchstone, J.; Moate, T.F.; Allen, E.H.; Kissel, J.C. Strategies for assessing children’s organophosphorus pesticide exposures in agricultural communities. J. Expo. Anal Environ. Epidemiol. 2020, 10, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garfitt, S.J.; Jones, K.; Mason, H.J.; Cocker, J. Exposure to the organophosphate diazinon: Data from a human volunteer study with oral and dermal doses. Toxico Lett. 2002, 134, 105–113. [Google Scholar] [CrossRef]
- Griffin, P.; Mason, H.; Heywood, K.; Cocker, J. Oral and dermal absorption of chlorpyrifos: A human volunteer study. Occup. Environ. Med. 1999, 56, 10–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Needham, L.L. Assessing exposure to organophosphorus pesticides by biomonitoring in epidemiologic studies of birth outcomes. Environ. Health Perspect. 2005, 113, 494–498. [Google Scholar] [CrossRef]
- Oya, N.; Ito, Y.; Ebara, T.; Kato, S.; Hioki, K.; Aoi, A.; Ueyama, J.; Oguri, T.; Shoji, N.; Sugiura-Ogasawara, M.; et al. Exposure levels of organophosphate pesticides in Japanese diapered children: Contributions of exposure-related behaviors and mothers’ considerations of food selection and preparation. Environ. Int. 2020, 134, 105294. [Google Scholar] [CrossRef]
- Hioki, K.; Ito, Y.; Oya, N.; Nakayama, S.F.; Isobe, T.; Ebara, T.; Shibata, K.; Nishikawa, N.; Nakai, K.; Kamida, T.; et al. Intra-individual variations of organophosphate pesticide metabolite concentrations in repeatedly collected urine samples from pregnant women in Japan. Environ. Health Prev. Med. 2019, 24, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S.EPA. Guidance on Cumulative Risk Assessment of Pesticide Chemicals That Have a Common Mechansim of Toxicity. 2002. Available online: https://www.epa.gov/sites/production/files/2015-07/documents/guidance_on_common_mechanism.pdf (accessed on 23 December 2020).
- U.S.EPA. Status of Cumulative Risk Assessment for Organophosphate Pesticides. 2002. Available online: www.epa.gov/pesticides/cumulative (accessed on 23 December 2020).
- U.S.EPA. Revised OP (Organophosphorate) Cumulative Risk Assessment. 2002. Available online: http://www.epa.gov/pesticides/cumulative%5Cnhttp://nepis.epa.gov/Exe/ZyNET.exe/9100BFLL.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2000+Thru+2005&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFiel (accessed on 23 December 2020).
- Castorina, R.; Bradman, A.; McKone, T.E.; Barr, D.B.; Harnly, M.E.; Eskenazi, B. Cumulative organophosphate pesticide exposure and risk assessment among pregnant women living in an agricultural community: A case study from the CHAMACOS cohort. Environ. Health Perspect. 2003, 111, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Yusà, V.; Muñoz, A.; Vera, T.; Borràs, E.; Ródenas, M.; Coscollà, C. Risk assessment of airborne pesticides in a Mediterranean region of Spain. Sci. Total Environ. 2017, 574, 724–734. [Google Scholar] [CrossRef] [PubMed]
- de Gavelle, E.; de Lauzon-Guillain, B.; Charles, M.A.; Chevrier, C.; Hulin, M.; Sirot, V.; Merlo, M.; Nougadère, A. Chronic dietary exposure to pesticide residues and associated risk in the French ELFE cohort of pregnant women. Environ. Int. 2016, 92–93, 533–542. [Google Scholar] [CrossRef]
- Hofmann, J.N.; Keifer, M.C.; De Roos, A.J.; Fenske, R.A.; Furlong, C.E.; Van Belle, G.; Checkoway, H. Occupational determinants of serum cholinesterase inhibition among organophosphate-exposed agricultural pesticide handlers in Washington State. Occup. Environ. Med. 2010, 67, 375–386. [Google Scholar] [CrossRef] [Green Version]
- London, L.; Myers, J.E. Use of a crop and job specific exposure matrix for retrospective assessment of long term exposure in studies of chronic neurotoxic effects of agrichemicals. Occup. Environ. Med. 1998, 55, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsikantami, I.; Colosio, C.; Alegakis, A.; Tzatzarakis, M.N.; Vakonaki, E.; Rizos, A.K.; Sarigiannis, D.A.; Tsatsakis, A.M. Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data—The internal exposure approach. Food Chem. Toxicol. 2019, 123, 57–71. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Sekiyama, M.; Yamazaki, S.; Michikawa, T.; Nakayama, S.F.; Nitta, H.; Taniguchi, Y.; Suda, E.; Isobe, T.; Kobayashi, Y.; Iwai-Shimada, M.; et al. Study design and participants’ profile in the Sub-Cohort Study in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2020. [Google Scholar] [CrossRef]
- Nishihama, Y.; Nakayama, S.F.; Tabuchi, T.; Isobe, T.; Jung, C.-R.; Iwai-Shimada, M.; Kobayashi, Y.; Michikawa, T.; Sekiyama, M.; Taniguchi, Y.; et al. Determination of Urinary Cotinine Cut-Off Concentrations for Pregnant Women in the Japan Environment and Children’s Study (JECS). Int. J. Environ. Res. Public Health 2020, 17, 5537. [Google Scholar] [CrossRef] [PubMed]
- Munch, D.; Branson, P. Statistical Protocol for the Determination of the Single-Laboratory Lowest Concentration Minimum Reporting Level (LCMRL) and Validation of Laboratory Performance at or Below the Minimum Reporting Level (MRL). Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P1005EAE.TXT?ZyActionD=ZyDocument&Client=EPA&Index=2000+Thru+2005&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery= (accessed on 16 February 2020).
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Iwai-Shimada, M.; Nakayama, S.F.; Isobe, T.; Michikawa, T.; Yamazaki, S.; Nitta, H.; Takeuchi, A.; Kobayashi, Y.; Tamura, K.; Suda, E.; et al. Questionnaire results on exposure characteristics of pregnant women participating in the Japan Environment and Children Study (JECS). Environ. Health Prev. Med. 2018, 23, 45. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Helsel, D.R. Fabricating data: How substituting values for nondetects can ruin results, and what can be done about it. Chemosphere 2006, 65, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, J.; Jia, E.; Chen, T.; Ni, Y.; Jia, W. GSimp: A Gibbs sampler based left-censored missing value imputation approach for metabolomics studies. PLoS Comput. Biol. 2018, 14, e1005973. [Google Scholar] [CrossRef] [Green Version]
- White, I.R.; Royston, P.; Wood, A.M. Multiple imputation using chained equations: Issues and guidance for practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef]
- Jung, C.-R.; Nishihama, Y.; Nakayama, S.F.; Tamura, K.; Isobe, T.; Michikawa, T.; Iwai-Shimada, M.; Kobayashi, Y.; Sekiyama, M.; Taniguchi, Y.; et al. Indoor Air Quality of 5000 Households and Its Determinants, Part B: Volatile Organic Compounds and Acidic Gasses in the Japan Environment and Children’s Study. Environ. Res. 2021, 197, 111135. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 23 December 2020).
- Arno, C.; Erin, L. Deep Learning with H2O, 6th ed.; Angela, B., Ed.; H2O. ai, Inc.: Mountain View, CA, USA, 2020; pp. 1–55. [Google Scholar]
- Yamada, T.; Kojima, T.; Akaishi, R.; Ishikawa, S.; Takeda, M.; Kawaguchi, S.; Nishida, R.; Morikawa, M.; Yamada, T.; Minakami, H. Problems in methods for the detection of significant proteinuria in pregnancy. J. Obstet. Gynaecol. Res. 2014, 40, 161–166. [Google Scholar] [CrossRef]
- World Health Organization Food and Agriculture Organization of the United Nations Pesticide residues in food 2007 Joint FAO/WHO Meeting on Pesticide Residues. Available online: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Report07/report2007jmpr.pdf (accessed on 8 January 2021).
- U.S.EPA. 2012; Benchmark Dose Technical Guidance. Available online: http://www.epa.gov/raf/publications/pdfs/benchmark_dose_guidance.pdf%5Cnpapers3://publication/uuid/368C9919-510C-4DF4-8DB7-118B49D7F4CA (accessed on 8 January 2021).
- Ein-Mor, E.; Ergaz-Shaltiel, Z.; Berman, T.; Göen, T.; Natsheh, J.; Ben-Chetrit, A.; Haimov-Kochman, R.; Calderon-Margalit, R. Decreasing urinary organophosphate pesticide metabolites among pregnant women and their offspring in Jerusalem: Impact of regulatory restrictions on agricultural organophosphate pesticides use? Int. J. Hyg. Environ. Health 2018, 221, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Pierik, F.H.; Angerer, J.; Meltzer, H.M.; Jaddoe, V.W.V.; Tiemeier, H.; Hoppin, J.A.; Longnecker, M.P. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Int. J. Hyg. Environ. Health 2009, 212, 481–491. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Tian, Y.; Wang, X.J.; Gao, Y.; Shi, R.; Wang, G.Q.; Hu, G.H.; Shen, X.M. Organophosphate pesticide exposure and perinatal outcomes in Shanghai, China. Environ. Int. 2012, 42, 100–104. [Google Scholar] [CrossRef]
- Lewis, R.C.; Cantonwine, D.E.; Anzalota Del Toro, L.V.; Calafat, A.M.; Valentin-Blasini, L.; Davis, M.D.; Montesano, M.A.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Distribution and determinants of urinary biomarkers of exposure to organophosphate insecticides in Puerto Rican pregnant women. Sci. Total Environ. 2015, 512–513, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdeen, Z.; Berman, T.; Azmi, K.; Abu Seir, R.; Agha, H.; Ein-Mor, E.; Göen, T.; Stein, Y.; Richter, E.; Calderon-Margalit, R. Urinary organophosphate metabolite levels in Palestinian pregnant women: Results of the Middle East Regional Cooperation Project. Int. J. Environ. Health Res. 2016, 26, 254–266. [Google Scholar] [CrossRef]
- Lu, C.; Bravo, R.; Caltabiano, L.M.; Irish, R.M.; Weerasekera, G.; Barr, D.B. The presence of dialkylphosphates in fresh fruit juices: Implication for organophosphorus pesticide exposure and risk assessments. J. Toxicol. Environ. Health-Part A 2005, 68, 209–227. [Google Scholar] [CrossRef]
- Quirós-Alcalá, L.; Bradman, A.; Smith, K.; Weerasekera, G.; Odetokun, M.; Barr, D.B.; Nishioka, M.; Castorina, R.; Hubbard, A.E.; Nicas, M.; et al. Organophosphorous pesticide breakdown products in house dust and children’s urine. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Funayama, K.; Komatus, M.; Sonoda, S.; Takahashi, I.; Hara, K. Management of apple orchards to conserve generalist phytoseiid mites suppresses two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2015, 65, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Handal, A.J.; Hund, L.; Páez, M.; Bear, S.; Greenberg, C.; Fenske, R.A.; Barr, D.B. Characterization of Pesticide Exposure in a Sample of Pregnant Women in Ecuador. Arch. Environ. Contam. Toxicol. 2016, 70, 627–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaan, S.; Pronk, A.; Koch, H.M.; Jusko, T.A.; Jaddoe, V.W.V.; Shaw, P.A.; Tiemeier, H.M.; Hofman, A.; Pierik, F.H.; Longnecker, M.P. Reliability of concentrations of organophosphate pesticide metabolites in serial urine specimens from pregnancy in the Generation R Study. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, M.; Basagaña, X.; Sakhi, A.K.; Haug, L.S.; Philippat, C.; Granum, B.; Manzano-Salgado, C.B.; Brochot, C.; Zeman, F.; de Bont, J.; et al. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ. Int. 2018, 121, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef] [Green Version]
| Statistics | Crude (ng/mL) | Creatinine-Normalised (µg/g-Creatinine) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMP | DMTP | DMDTP | DEP | DETP | DEDTP c | DMP | DMTP | DMDTP | DEP | DETP | DEDTP | |
| DR (%) | 80.8 | 80.0 | 16.1 | 81.2 | 22.9 | 0.02 | 80.8 | 80.0 | 16.1 | 81.2 | 22.9 | 0.02 |
| Mean | 5.69 | 11.3 | - | 5.12 | - | - | 5.79 | 11.8 | - | 5.32 | - | - |
| SD | 10.4 | 45.6 | - | 8.97 | - | - | 8.07 | 31.5 | - | 9.54 | - | - |
| Min | <MRL a | <MRL a | <MRL a | <MRL a | <MRL b | - | <MRL | - | ||||
| 25th | 1.33 | 1.32 | - | 1.32 | - | - | 1.91 | 1.66 | - | 1.88 | - | - |
| 50th | 2.93 | 3.29 | - | 2.78 | - | - | 3.53 | 4.09 | - | 3.28 | - | - |
| 75th | 6.16 | 9.38 | - | 5.47 | - | - | 6.78 | 10.8 | - | 5.98 | - | - |
| 95th | 18.6 | 40.2 | 2.94 | 16.1 | 4.50 | - | 17.8 | 44.5 | 3.22 | 15.3 | 5.15 | - |
| Max | 385 | 1640 | 95.4 | 213 | 319 | 34.9 | 226 | 891 | 50.4 | 380 | 681 | 126 |
| Statistics | DAPs | DMs | DEs |
|---|---|---|---|
| Mean | 0.88 | 0.82 | 0.058 |
| SD | 1.72 | 1.69 | 0.13 |
| GM | 0.47 | 0.42 | 0.036 |
| GSD | 2.82 | 2.98 | 2.52 |
| Min | 0.011 | 0.0042 | 0.0011 |
| 25th | 0.23 | 0.20 | 0.019 |
| 50th | 0.45 | 0.40 | 0.035 |
| 75th | 0.92 | 0.84 | 0.063 |
| 95th | 2.90 | 2.78 | 0.17 |
| Max | 48.7 | 47.8 | 5.50 |
| >BMDL10/100 a —n, (%) | 16 (0.36) | 16 (0.36) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishihama, Y.; Nakayama, S.F.; Isobe, T.; Jung, C.-R.; Iwai-Shimada, M.; Kobayashi, Y.; Michikawa, T.; Sekiyama, M.; Taniguchi, Y.; Yamazaki, S.; et al. Urinary Metabolites of Organophosphate Pesticides among Pregnant Women Participating in the Japan Environment and Children’s Study (JECS). Int. J. Environ. Res. Public Health 2021, 18, 5929. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18115929
Nishihama Y, Nakayama SF, Isobe T, Jung C-R, Iwai-Shimada M, Kobayashi Y, Michikawa T, Sekiyama M, Taniguchi Y, Yamazaki S, et al. Urinary Metabolites of Organophosphate Pesticides among Pregnant Women Participating in the Japan Environment and Children’s Study (JECS). International Journal of Environmental Research and Public Health. 2021; 18(11):5929. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18115929
Chicago/Turabian StyleNishihama, Yukiko, Shoji F. Nakayama, Tomohiko Isobe, Chau-Ren Jung, Miyuki Iwai-Shimada, Yayoi Kobayashi, Takehiro Michikawa, Makiko Sekiyama, Yu Taniguchi, Shin Yamazaki, and et al. 2021. "Urinary Metabolites of Organophosphate Pesticides among Pregnant Women Participating in the Japan Environment and Children’s Study (JECS)" International Journal of Environmental Research and Public Health 18, no. 11: 5929. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18115929






