The Effect of Bilateral Nephrectomy on Renalase and Catecholamines in Hemodialysis Patients
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients
2.2. Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Li, G.; Wang, P.; Velazquez, H.; Yao, X.; Li, Y.; Wu, Y.; Peixoto, A.; Crowley, S.; Desir, G.V. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J. Clin. Investig. 2005, 115, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Desir, G.V.; Tang, L.; Wang, P.; Li, G.; Sampaio-Maia, B.; Quelhas-Santos, J.; Pestana, M.; Velazquez, H. Renalase Lowers Ambulatory Blood Pressure by Metabolizing Circulating Adrenaline. J. Am. Hear. Assoc. 2012, 1, e002634. [Google Scholar] [CrossRef] [Green Version]
- Beaupre, B.A.; Carmichael, B.R.; Hoag, M.R.; Shah, D.D.; Moran, G.R. Renalase Is an α-NAD(P)H Oxidase/Anomerase. J. Am. Chem. Soc. 2013, 135, 13980–13987. [Google Scholar] [CrossRef]
- Milani, M.; Ciriello, F.; Baroni, S.; Pandini, V.; Canevari, G.; Bolognesi, M.; Aliverti, A. FAD-Binding Site and NADP Reactivity in Human Renalase: A New Enzyme Involved in Blood Pressure Regulation. J. Mol. Biol. 2011, 411, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Beaupre, B.A.; Roman, J.V.; Hoag, M.R.; Meneely, K.M.; Silvaggi, N.R.; Lamb, A.; Moran, G.R. Ligand binding phenomena that pertain to the metabolic function of renalase. Arch. Biochem. Biophys. 2016, 612, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Moran, G.R.; Hoag, M.R. The enzyme: Renalase. Arch. Biochem. Biophys. 2017, 632, 66–76. [Google Scholar] [CrossRef]
- Wang, L.; Velazquez, H.; Chang, J.; Safirstein, R.; Desir, G.V. Identification of a Receptor for Extracellular Renalase. PLoS ONE 2015, 10, e0122932. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Velazquez, H.; Moeckel, G.; Chang, J.; Ham, A.; Lee, H.T.; Safirstein, R.; Desir, G.V. Renalase Prevents AKI Independent of Amine Oxidase Activity. J. Am. Soc. Nephrol. 2014, 25, 1226–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Liu, X.; Zhao, T.; Liang, R.; Wu, R.; Zhang, F.; Kong, Y.; Liu, L.; Xing, T.; Wang, N.; et al. A protective role of renalase in diabetic nephropathy. Clin. Sci. 2020, 134, 75–85. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Q.; Yuan, Y.; Zhang, C.; Wu, L.; Liu, X.; Cao, W.; Guo, H.; Duan, S.; Xu, X.; et al. Renalase attenuates mitochondrial fission in cisplatin-induced acute kidney injury via modulating sirtuin-3. Life Sci. 2019, 222, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Deng, D.; Zhang, Q.; Liu, W. Renalase Protects against Renal Fibrosis by Inhibiting the Activation of the ERK Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 855. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Zhao, Q.; Li, J.; Xing, T.; Wang, F.; Wang, N. Renalase Protects against Contrast-Induced Nephropathy in Sprague-Dawley Rats. PLoS ONE 2015, 10, e0116583. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Kim, J.Y.; Kim, M.; Wang, P.; Tang, L.; Baroni, S.; D’Agati, V.D.; Desir, G.V. Renalase Protects against Ischemic AKI. J. Am. Soc. Nephrol. 2013, 24, 445–455. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Desir, G.V. Renalase, a new renal hormon: Its role in health and disease. Curr. Opin. Nephrol. Hypertens. 2007, 16, 373–378. [Google Scholar] [CrossRef]
- Li, G.; Xu, J.; Wang, P.; Velazquez, H.; Li, Y.; Wu, Y.; Desir, G.V. Catecholamines Regulate the Activity, Secretion, and Synthesis of Renalase. Circulation 2008, 117, 1277–1282. [Google Scholar] [CrossRef]
- Baek, S.H.; Cha, R.-H.; Kang, S.W.; Park, C.W.; Cha, D.R.; Kim, S.G.; Yoon, S.A.; Kim, S.; Han, S.-Y.; Park, J.H.; et al. Circulating renalase predicts all-cause mortality and renal outcomes in patients with advanced chronic kidney disease. Korean J. Intern. Med. 2019, 34, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Desir, G.V.; Peixoto, A.J. Renalase in hypertension and kidney disease. Nephrol. Dial. Transplant. 2013, 29, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciorkowska, D.; Zbroch, E.; Malyszko, J. Circulating renalase, catecholamines, and vascular adhesion protein 1 in hypertensive patients. J. Am. Soc. Hypertens. 2015, 9, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Velazquez, H.; Safirstein, R.; Desir, G.V. Renalase: Its role as a cytokine, and an update on its association with type 1 diabetes and ischemic stroke. Curr. Opin. Nephrol. Hypertens. 2014, 23, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, R.; Xie, Z.; Lin, M.; Liang, Z.; Yang, Y.; Jiang, W. Renalase, a new secretory enzyme: Its role in hypertensive-ischemic cardiovascular diseases. Med. Sci. Monit. 2014, 20, 688–692. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Huang, B.; Li, J.; Liu, L.; Wang, N. Renalase might be associated with hypertension and insulin resistance in Type 2 diabetes. Ren. Fail. 2014, 36, 552–556. [Google Scholar] [CrossRef]
- Zbroch, E.; Koc-Zorawska, E.; Malyszko, J.; Malyszko, J.; Mysliwiec, M. Circulating Levels of Renalase, Norepinephrine, and Dopamine in Dialysis Patients. Ren. Fail. 2013, 35, 673–679. [Google Scholar] [CrossRef]
- Zbroch, E.; Malyszko, J.; Koc-Zorawska, E.; Mysliwiec, M. Renalase, a Novel Enzyme Involved in Blood Pressure Regulation, Is Related to Kidney Function but Not to Blood Pressure in Hemodialysis Patients. Kidney Blood Press. Res. 2012, 35, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, M.; Serwin, N.; Dziedziejko, V.; Marchelek-Myśliwiec, M.; Dołęgowska, B.; Domański, L.; Ciechanowski, K.; Safranow, K.; Pawlik, A. Chronic kidney disease is associated with increased levels of renalase in serum and decreased in erythrocytes. Pol. Arch. Intern. Med. 2019, 129, 790–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisniewska, M.; Serwin, N.; Dziedziejko, V.; Marchelek-Mysliwiec, M.; Dołegowska, B.; Domanski, L.; Ciechanowski, K.; Safranow, K.; Pawlik, A. Renalase in Haemodialysis Patients with Chronic Kidney Disease. J. Clin. Med. 2021, 10, 680. [Google Scholar] [CrossRef]
- Li, J.-H.; Luo, J.-F.; Jiang, Y.; Ma, Y.-J.; Ji, Y.-Q.; Zhu, G.-L.; Zhou, C.; Chu, H.-W.; Zhang, H.-D. Red Blood Cell Lifespan Shortening in Patients with Early-Stage Chronic Kidney Disease. Kidney Blood Press. Res. 2019, 44, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Shastry, I.; Belurkar, S. The spectrum of red blood cell parameters in chronic kidney disease: A study of 300 cases. J. Appl. Hematol. 2019, 10, 61–66. [Google Scholar] [CrossRef]
- Karsten, E.; Breen, E.; Herbert, B.R. Red blood cells are dynamic reservoirs of cytokines. Sci. Rep. 2018, 8, 3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurya, P.K.; Kumar, P.; Chandra, P. Biomarkers of oxidative stress in erythrocytes as a function of human age. World J. Methodol. 2015, 5, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taranta-Janusz, K.; Roszkowska, R.; Wasilewska, A. Renalase Levels in Children with Solitary Functioning Kidney. Indian Pediatr. 2015, 52, 1047–1050. [Google Scholar] [CrossRef]
- Yin, J.; Lu, Z.; Wang, F.; Jiang, Z.; Lu, L.; Miao, N.; Wang, N. Renalase attenuates hypertension, renal injury and cardiac remodelling in rats with subtotal nephrectomy. J. Cell. Mol. Med. 2016, 20, 1106–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraka, A.; El Ghotny, S. Cardioprotective Effect of Renalase in 5/6 Nephrectomized Rats. J. Cardiovasc. Pharmacol. Ther. 2012, 17, 412–416. [Google Scholar] [CrossRef]
- Malyszko, J.; Koc-Zorawska, E.; Zorawski, M.; Kozminski, P.; Zbroch, E.; Rysz, J.; Banach, M.; Malyszko, J.S. Renalase is removed by kidneys and during dialysis – excess related to CKD complications? Curr. Vasc. Pharmacol. 2015, 13, 134–140. [Google Scholar] [CrossRef]
- Stojanovic, D.; Cvetkovic, T.; Stojanovic, M.; Stefanovic, N.; Velickovic-Radovanovic, R.; Zivkovic, N. Renalase Assessment With Regard to Kidney Function, Lipid Disturbances, and Endothelial Dysfunction Parameters in Stable Renal Transplant Recipients. Prog. Transplant. 2017, 27, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zbroch, E.; Małyszko, J.; Małyszko, J.; Koc-Żórawska, E.; Myśliwiec, M. Renalase, kidney function, and markers of endo-thelial dysfunction in renal transplantrecipients. Pol. Arch. Med. Wewn. 2012, 122, 40–44. [Google Scholar]
- Przybylowski, P.; Kozlowska, S.; Malyszko, J.; Koc-Zorawska, E.; Myśliwiec, M. Serum Renalase Depends on Kidney Function But Not on Blood Pressure in Heart Transplant Recipients. Transplant. Proc. 2011, 43, 3888–3891. [Google Scholar] [CrossRef] [PubMed]
- Przybylowski, P.; Koc-Zorawska, E.; Malyszko, J.; Myśliwiec, M.; Malyszko, J. Renalase and Endothelial Dysfunction in Heart Transplant Recipients. Transplant. Proc. 2013, 45, 394–396. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, Y.; Tan, L.; Tang, X.; Yang, Q.; Yang, K. Impact of renal denervation on renalase expression in adult rats with spontaneous hypertension. Exp. Ther. Med. 2012, 4, 493–496. [Google Scholar] [CrossRef] [Green Version]
- Lemiesz, M.; Tenderenda-Banasiuk, E.; Sosnowska, D.; Taranta-Janusz, K.; Wasilewska, A. Serum Renalase Levels in Adolescents with Primary Hypertension. Pediatr. Cardiol. 2018, 39, 1258–1264. [Google Scholar] [CrossRef] [Green Version]
- Malyszko, J.; Koc-Zorawska, E.; Malyszko, J.S.; Kozminski, P.; Zbroch, E.; Mysliwiec, M. Renalase, Stroke, and Hypertension in Hemodialyzed Patients. Ren. Fail. 2012, 34, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Malyszko, J.; Malyszko, J.S.; Rysz, J.; Mysliwiec, M.; Tesar, V.; Levin-Iaina, N.; Banach, M. Renalase, Hypertension, and Kidney—The Discussion Continues. Angiology 2013, 64, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zbroch, E.; Małyszko, J.; Małyszko, J.; Żórawski, M.J.; Myśliwiec, M. Kidney and hypertension: Is there a place for renalase? Pol. Arch. Intern. Med. 2012, 122, 174–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desir, G.V.; Wang, L.; Peixoto, A.J. Human renalase: A review of its biology, function, and implications for hypertension. J. Am. Soc. Hypertens. 2012, 6, 417–426. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Patients Post Bilateral Nephrectomy | Healthy Controls | Hemodialysis Patients with Anuria | Patients Post Bilateral Nephrectomy vs. Healthy Controls | Patients Post Bilateral Nephrectomy vs. Hemodialysis Patients with Anuria | Healthy Controls vs. Hemodialysis Patients with Anuria |
|---|---|---|---|---|---|---|
| Mean ± SD | p * | |||||
| Age (years) | 52.5 ± 13.5 | 57.4 ± 18.5 | 66.2 ± 16.8 | 0.52 | <0.01 | 0.038 |
| Glucose (mg/dL) | 98.2 ± 15.6 | 86.7 ± 7.2 | 104.1 ± 31.6 | <0.01 | 0.85 | <0.01 |
| Creatinine (mg/dL) | 7.10 ± 3.80 | 0.83 ± 0.10 | 8.51 ± 3.68 | <0.01 | 0.060 | <0.01 |
| Uric acid (mg/dL) | 5.0 ± 2.8 | 5.5 ± 0.6 | 7.1 ± 1.7 | 0.033 | <0.01 | <0.01 |
| Total protein in serum (g/L) | 7.54 ± 1.07 | 7.44 ± 0.43 | 5.70 ± 0.86 | 0.85 | <0.01 | <0.01 |
| Albumin (g/L) | 3.75 ± 0.63 | 3.70 ± 0.16 | 3.16 ± 0.51 | 0.44 | <0.01 | <0.01 |
| HGB (mmol/L) | 6.3 ± 0.6 | 7.9 ± 0.6 | 6.8 ± 1.1 | <0.01 | 0.033 | <0.01 |
| RBC (T/L) | 3.6 ± 0.4 | 4.8 ± 0.4 | 3.6 ± 0.6 | <0.01 | 0.77 | <0.01 |
| MCHC (mmol/L) | 20.3 ± 0.5 | 19.5 ± 0.6 | 20.6 ± 0.6 | <0.01 | 0.023 | <0.01 |
| Adrenaline (pg/mL) | 65.4 ± 129.1 | 30.4 ± 33.9 | 15.3 ± 12.9 | 0.11 | <0.01 | 0.039 |
| Noradrenaline (pg/mL) | 413 0 ± 579.0 | 399.1 ± 319.0 | 301.0 ± 498.2 | 0.22 | 0.47 | <0.01 |
| Dopamine (pg/mL) | 116.8 ± 147.7 | 440.9 ± 343.2 | 179.0 ± 125.2 | <0.01 | <0.01 | <0.01 |
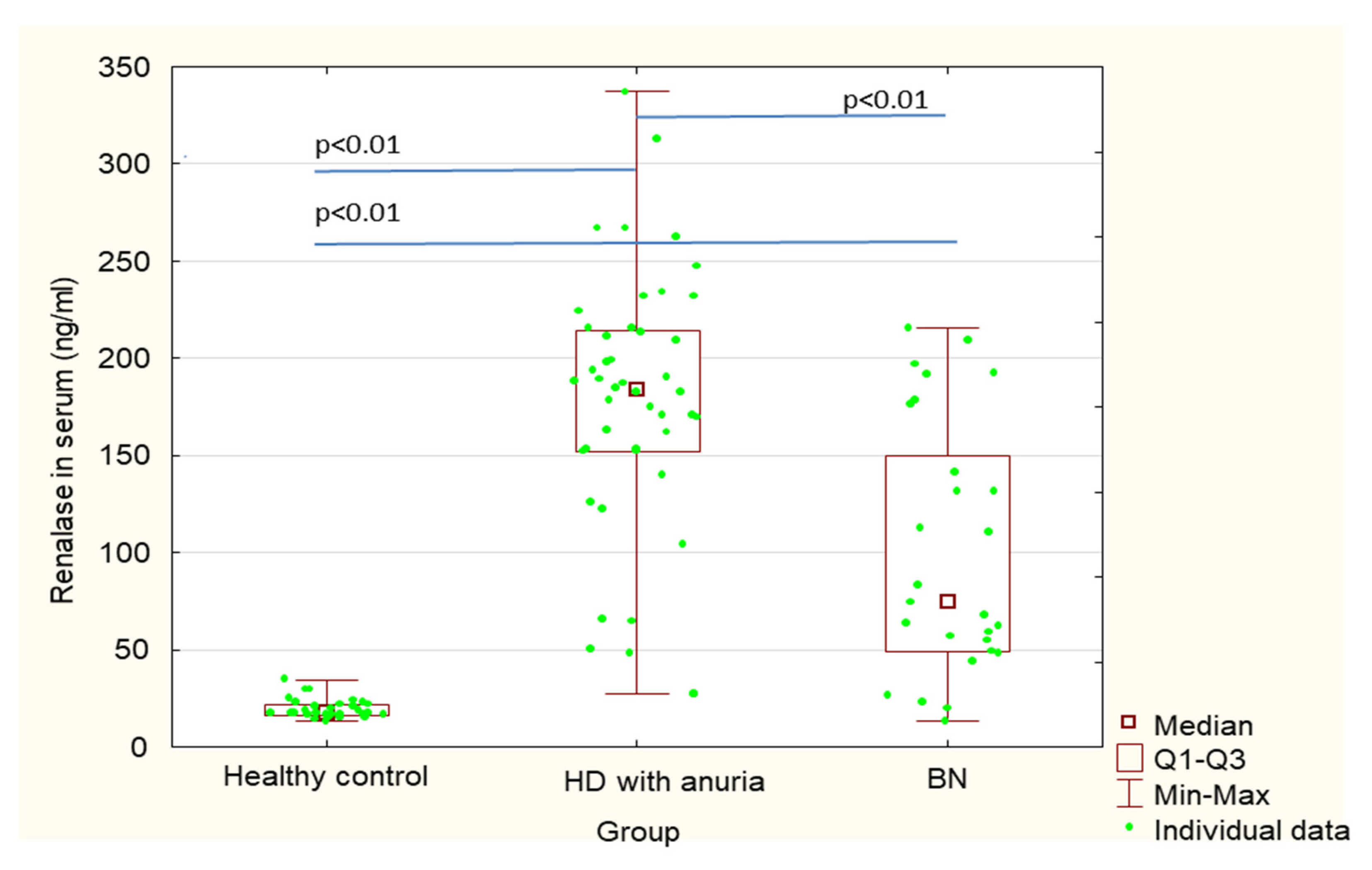
| Renalase in serum (ng/mL) | 101.1 ± 65.5 | 19.6 ± 5.0 | 177.2 ± 68.3 | <0.01 | <0.01 | <0.01 |
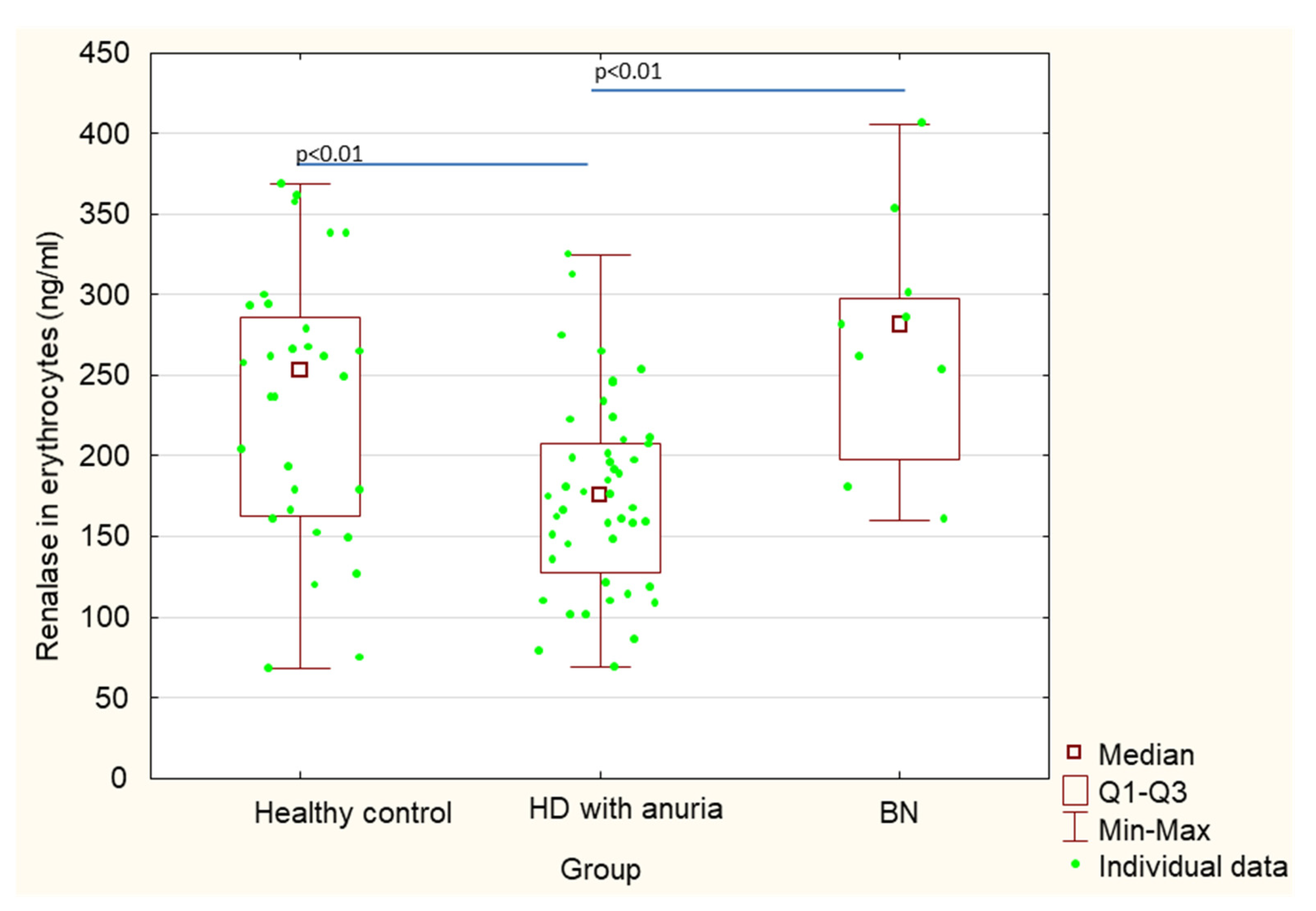
| Renalase in erythrocytes (ng/mL) | 275.8 ± 76.7 | 233.2 ± 83.1 | 176.4 ± 59.1 | 0.18 | <0.01 | <0.01 |
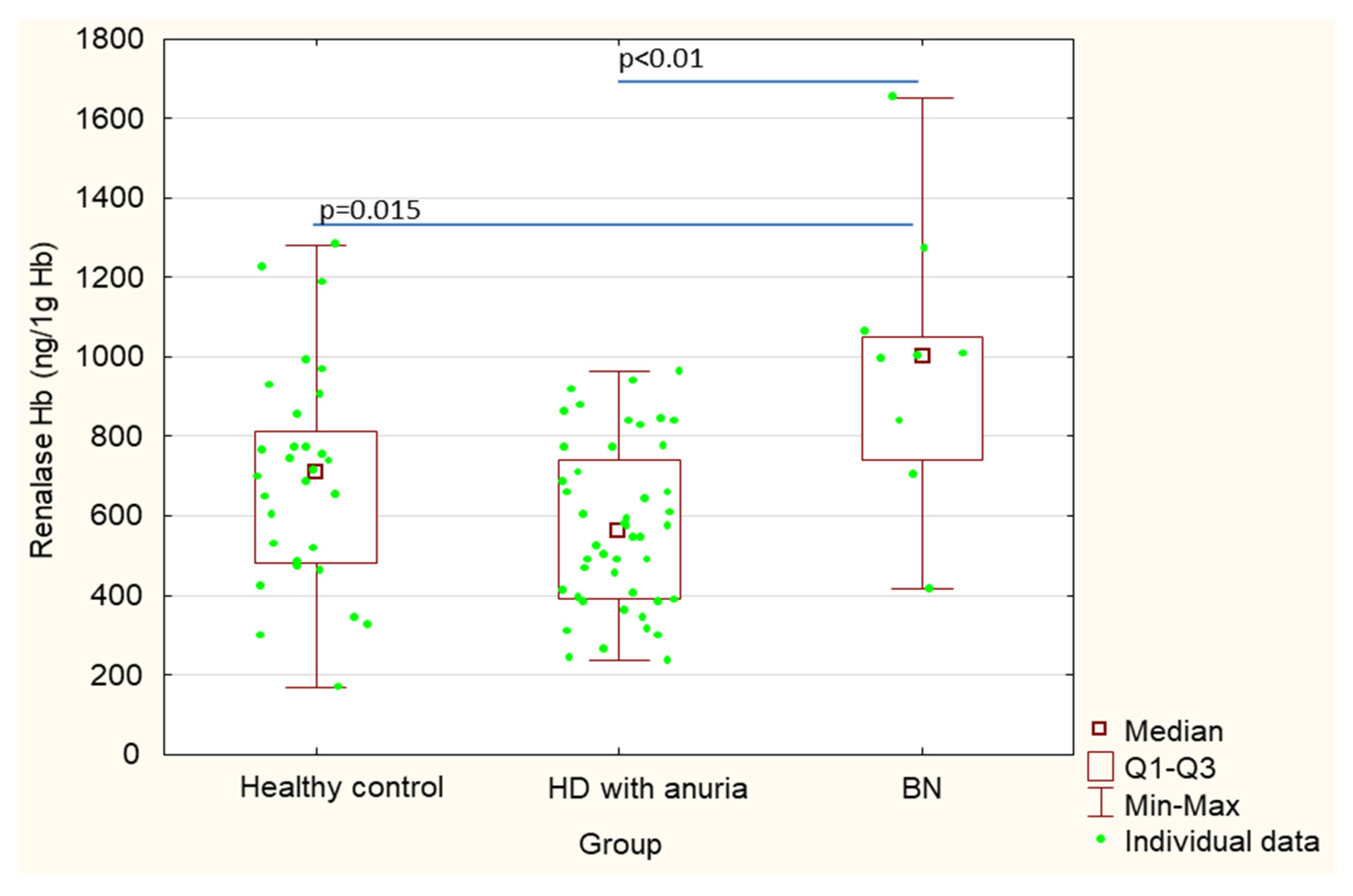
| Renalase Hb (ng/1 g Hb) | 994.9 ± 345.5 | 697.6 ± 273.4 | 573.1 ± 205.8 | 0.015 | <0.01 | 0.060 |
| Parameters | Renalase in Serum | Renalase in Erythrocytes | Renalase Hb | |||
|---|---|---|---|---|---|---|
| Rs | p | Rs | p | Rs | p | |
| Renalase in erythrocytes | −0.03 | 0.93 | - | - | - | - |
| Renalase Hb | −0.02 | 0.97 | 0.93 | 0.0002 | - | - |
| Glucose | −0.02 | 0.92 | −0.54 | 0.14 | −0.46 | 0.21 |
| Uric acid | −0.31 | 0.12 | −0.38 | 0.31 | −0.37 | 0.33 |
| Total protein in serum | −0.41 | 0.03 | 0.60 | 0.08 | 0.50 | 0.17 |
| Albumin | −0.34 | 0.084 | 0.78 | 0.01 | 0.65 | 0.05 |
| HGB | 0.11 | 0.59 | 0.27 | 0.49 | 0.19 | 0.62 |
| RBC | 0.08 | 0.68 | 0.30 | 0.43 | 0.23 | 0.55 |
| MCHC | 0.15 | 0.44 | 0.82 | 0.006 | 0.82 | 0.007 |
| Adrenaline | 0.04 | 0.87 | 0.13 | 0.73 | −0.02 | 0.97 |
| Noradrenaline | −0.10 | 0.62 | 0.25 | 0.52 | 0.17 | 0.67 |
| Dopamine | −0.01 | 0.96 | 0.17 | 0.67 | 0.27 | 0.49 |
| Duration of hemodialysis | −0.18 | 0.38 | −0.32 | 0.41 | −0.37 | 0.33 |
| Parameters | Patients Post-Bilateral Nephrectomy | Healthy Controls | Hemodialysis Patients with Anuria | ||||||
|---|---|---|---|---|---|---|---|---|---|
| without HA | with HA | p * | without HA | with HA | p * | without HA | with HA | p * | |
| Mean ± SD | Mean ± SD | Mean ± SD | |||||||
| Renalase in serum (ng/mL) | 109.4 ± 73.0 | 98.2 ± 61.9 | 0.91 | 18.1 ± 3.8 | 23.3 ± 5.8 | 0.007 | 167.9 ± 59.1 | 178.3 ± 69.8 | 1.00 |
| Renalase in erythrocytes (ng/mL) | 279.0 ± 7.5 | 273.3 ± 88.1 | 0.62 | 217.7 ± 75.0 | 269.6 ± 94.2 | 0.07 | 173.1 ± 63.8 | 176.8 ± 59.3 | 0.99 |
| Renalase Hb (ng/1 g Hb) | 936.6 ± 366.6 | 1041.5 ± 363.2 | 0.63 | 640.7 ± 240.7 | 830.5 ± 312.8 | 0.09 | 579.3 ± 289.4 | 572.3 ± 198.2 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, M.; Serwin, N.; Dziedziejko, V.; Marchelek-Myśliwiec, M.; Dołęgowska, B.; Domański, L.; Ciechanowski, K.; Safranow, K.; Gołębiowski, T.; Pawlik, A. The Effect of Bilateral Nephrectomy on Renalase and Catecholamines in Hemodialysis Patients. Int. J. Environ. Res. Public Health 2021, 18, 6282. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18126282
Wiśniewska M, Serwin N, Dziedziejko V, Marchelek-Myśliwiec M, Dołęgowska B, Domański L, Ciechanowski K, Safranow K, Gołębiowski T, Pawlik A. The Effect of Bilateral Nephrectomy on Renalase and Catecholamines in Hemodialysis Patients. International Journal of Environmental Research and Public Health. 2021; 18(12):6282. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18126282
Chicago/Turabian StyleWiśniewska, Magda, Natalia Serwin, Violetta Dziedziejko, Małgorzata Marchelek-Myśliwiec, Barbara Dołęgowska, Leszek Domański, Kazimierz Ciechanowski, Krzysztof Safranow, Tomasz Gołębiowski, and Andrzej Pawlik. 2021. "The Effect of Bilateral Nephrectomy on Renalase and Catecholamines in Hemodialysis Patients" International Journal of Environmental Research and Public Health 18, no. 12: 6282. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18126282






