Environmental Impact Assessment in the Former Mining Area of Regoufe (Arouca, Portugal): Contributions to Future Remediation Measures
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
3.1. Sampling
3.2. Sample Processing
3.3. Analysis
3.3.1. Determination of Soil Mineralogy
3.3.2. Physicochemical Parameters of Soils and Waters
3.3.3. Chemical Analysis of Soils and Waters
3.3.4. Sequential Selective Chemical Extraction (SSCE)
3.4. Data Processing and Statistical Approaches
4. Results and Discussion
4.1. Soils
4.2. Waters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adriano, D.C. Trace Elements in Terrestrial Environments–Biogeochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; p. 867. [Google Scholar]
- Siegel, F.R. Environmental Geochemistry of Potentially Toxic Heavy Metals; Springer: Heidelberg, Germany, 2002; p. 218. [Google Scholar]
- Callender, E. Heavy metals in the environment–Historical trends. In Environmental Geochemistry; Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Lollar, B.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9, pp. 67–105. [Google Scholar]
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment, Environmental Impacts, 2nd ed.; Springer: Berlin, Germany, 2007; p. 304. [Google Scholar]
- Querol, X.; Alastuey, A.; Lopez-Soler, A.; Plana, F. Levels and chemistry of atmospheric particulates induced by a spill of heavy metal mining wastes in the Doñana area, Southwest Spain. Atmos. Environ. 2000, 34, 239–253. [Google Scholar] [CrossRef]
- Lim, H.-S.; Lee, J.-S.; Chon, H.-T.; Sager, M. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. J. Geochem. Explor. 2008, 96, 223–230. [Google Scholar] [CrossRef]
- Navarro, M.; Perezsirvent, C.; Martínez-Sánchez, M.; Vidal, J.; Tovar, P.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Del Rio-Salas, R.; Ayala-Ramírez, Y.; Loredo-Portales, R.; Romero, F.; Molina-Freaner, F.; Minjarez-Osorio, C.; Pi-Puig, T.; Ochoa–Landín, L.; Moreno-Rodríguez, V. Mineralogy and Geochemistry of Rural Road Dust and Nearby Mine Tailings: A Case of Ignored Pollution Hazard from an Abandoned Mining Site in Semi-arid Zone. Nat. Resour. Res. 2019, 28, 1485–1503. [Google Scholar] [CrossRef]
- Leblanc, M.; Morales, J.A.; Borrego, J.; Elbaz-Poulichet, F. 4,500-Year-old mining pollution in southwestern Spain: Long-term implications for modern mining pollution. Econ. Geol. 2000, 95, 655–662. [Google Scholar] [CrossRef]
- Moncur, M.; Ptacek, C.; Blowes, D.; Jambor, J. Release, transport and attenuation of metals from an old tailings impoundment. Appl. Geochem. 2005, 20, 639–659. [Google Scholar] [CrossRef]
- Santos Oliveira, J.M.; Farinha, J.; Matos, J.X.; Ávila, P.; Rosa, C.; Canto Machado, M.J.; Daniel, F.S.; Martins, L.; Machado Leite, M.R. Diagnóstico ambiental das principais áreas mineiras degradadas do país. Bol. Minas 2002, 39, 67–85. [Google Scholar]
- Ferreira da Silva, E.; Zhang, C.; Pinto, L.S.; Patinha, C.; Reis, P. Hazard assessment on arsenic and lead in soils of Castromil gold mining area, Portugal. Appl. Geochem. 2004, 19, 887–898. [Google Scholar] [CrossRef]
- Álvarez-Valero, A.M.; Pérez-López, R.; Matos, J.; Capitán, M.A.; Nieto, J.M.; Sáez, R.; Delgado, J.; Caraballo, M. Potential environmental impact at São Domingos mining district (Iberian Pyrite Belt, SW Iberian Peninsula): Evidence from a chemical and mineralogical characterization. Environ. Earth Sci. 2008, 55, 1797–1809. [Google Scholar] [CrossRef]
- Neiva, A.; Antunes, M.; Carvalho, P.C.S.; Santos, A. Uranium and arsenic contamination in the former Mondego Sul uranium mine area, Central Portugal. J. Geochem. Explor. 2016, 162, 1–15. [Google Scholar] [CrossRef]
- Abreu, M.M.; Matias, M.; Magalhães, M.C.F.; Basto, M. Impacts on water, soil and plants from the abandoned Miguel Vacas copper mine, Portugal. J. Geochem. Explor. 2008, 96, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Antunes, M.; Gomes, M.; Neiva, A.; Carvalho, P.; Santos, A. Potential risk assessment in stream sediments, soils and waters after remediation in an abandoned W>Sn mine (NE Portugal). Ecotoxicol. Environ. Saf. 2016, 133, 135–145. [Google Scholar] [CrossRef]
- Julivert, M.; Fontboté, J.; Ribeiro, A.; Conde, L. Memoria Explicativa del Mapa Tectónico de la Península Ibérica y Baleares (Escala 1:1000000); Instituto Geológico y Minero de España: Madrid, Spain, 1974; p. 113. [Google Scholar]
- Silva, A.; Rebelo, J.; Ribeiro, M.L. Notícia Explicativa da Carta Geológica de Portugal na Escala 1:50000 da Folha 11C (Torre de Moncorvo); Serviços Geológicos de Portugal: Lisboa, Portugal, 1989; p. 65. [Google Scholar]
- Ferreira, N.; Iglesias, M.; Noronha, F.; Pereira, E.; Ribeiro, A.; Ribeiro, M. Granitóides da Zona Centro Ibérica e seu enquadramento geodinâmico. In Libro Homenaje a L.C. Garcia de Figueirola, Geologia de Los Granitoides y Rocas Associadas del Macizo Hesperico; Bea, F., Carnicero, A., Gonzalo, J.C., Lópes Plaza, M., Rodríguez Alonso, M.D., Eds.; Editorial Rueda: Madrid, Spain, 1987; pp. 37–51. [Google Scholar]
- Sluijk, D. Geology and Tin-Tungsten Deposits of the Regoufe Area, Northern Portugal. Ph.D. Thesis, Geologisch Instituut, Mededeling, Universiteit van Amsterdam, Amsterdam, The Netherlands, 12 June 1963. [Google Scholar]
- Pereira, E.; Rodrigues, J.; Gonçalves, L.S.M.; Moreira, A.; Silva, A.F. Carta Geológica de Portugal Na escala 1:50.000 & Notícia Explicativa da Folha 13D (Oliveira de Azeméis); Instituto Nacional de Engenharia, Tecnologia e Inovação: Lisboa, Portugal, 2007; p. 55. [Google Scholar]
- Pinto, M.S. Granitóides dos maciços de Arouca e Regoufe: Dados geoquímicos e isotópicos e algumas implicações. Comun. Serv. Geol. Port. 1985, 71, 159–169. [Google Scholar]
- Vriend, S.; Oosterom, M.; Bussink, R.; Jansen, J. Trace-element behavior in the W–Sn granite of Regoufe, Portugal. J. Geochem. Explor. 1985, 23, 13–25. [Google Scholar] [CrossRef]
- Van Gaans, P.; Vriend, S.; Poorter, R. Hydrothermal processes and shifting element association patterns in the W—Sn enriched granite of Regoufe, Portugal. J. Geochem. Explor. 1995, 55, 203–222. [Google Scholar] [CrossRef]
- Van De Haar, A.; Vriend, S.; Van Gaans, P. Hydrothermal alteration of the Beira schists around the W-Sn specialised Regoufe granite, NW Portugal. J. Geochem. Explor. 1993, 46, 335–347. [Google Scholar] [CrossRef]
- Favas, P.J.C. Biogeoquímica em áreas Mineiras Estano-Volframíticas. Ph.D. Thesis, Universidade de Trás-os-Montes e Alto Douro, Vila Real, Portugal, 28 July 2008. [Google Scholar]
- Jacques, D.; Muchez, P.; Sintubin, M. Superimposed folding and W-Sn vein-type mineralisation in the Central Iberian Zone associated with late-Variscan oroclinal buckling: A structural analysis from the Regoufe area (Portugal). Tectonphysics 2018, 742, 66–83. [Google Scholar] [CrossRef]
- ISO 10390. Standard of Soil Quality–Determination of pH; International Organization for Standardization: Geneva, Switzerland, 1994. [Google Scholar]
- ISO 13536. Soil Quality–Determination of the Potential Cation Exchange Capacity and Exchangeable Cations Using Barium Chloride Solution Buffered at pH = 8.1; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Schumacher, B.A. Methods for the Determination of Total Organic Carbon (TOC) in Soils and Sediments; Ecological Risk Assessment Support Center, Environmental Protection Agency: Washington, DC, USA, 2002; p. 23.
- Fonseca, E.C.; Martin, H. The selective extraction of Pb and Zn in selected mineral and soil samples, application in geochemical exploration (Portugal). J. Geochem. Explor. 1986, 26, 231–248. [Google Scholar] [CrossRef]
- Cardoso Fonseca, E.; Ferreira da Silva, E.; Martins, M.E.; Patinha, C.; Moreno, F.; Reis, A.P. Extracção química selectiva, Princípios e problemas. Geociências. Rev. Univ. Aveiro 1999, 13, 45–57. [Google Scholar]
- Soil Science Division Staff. Soil survey manual. In USDA Handbook 18; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; Government Printing Office: Washington, DC, USA, 2017; p. 603. [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis; Springer Science and Business Media LLC: Berlin, Germany, 2006; p. 993. [Google Scholar]
- Bailey, J.S.; Stevens, R.J.; Kilpatrick, D.J. A rapid method for predicting the lime requirement of acidic temperate soils with widely varying organic matter contents. I. Development of the lime requirement model. J. Soil Sci. 1989, 40, 807–820. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Fonseca, B. Avaliação da Contaminação Associada às Escombreiras das Minas de Rio de Frades–Arouca. Master’s Thesis, Faculdade de Ciências da Universidade do Porto, Porto, Portugal, 26 July 2019. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2011; p. 505. [Google Scholar]
- Ferreira, M. Dados Geoquímicos de Base de Solos de Portugal Continental, Utilizando Amostragem de Baixa Densidade. Ph.D. Thesis, University of Aveiro, Aveiro, Portugal, 19 December 2004. [Google Scholar]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, B. Defining and modeling the soil geochemical background of heavy metals from the Hengshi River watershed (southern China): Integrating EDA, stochastic simulation and magnetic parameters. J. Hazard. Mat. 2010, 180, 542–551. [Google Scholar] [CrossRef]
- He, Z.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Koutsospyros, A.; Braida, W.; Christodoulatos, C.; Dermatas, D.; Strigul, N. A review of tungsten: From environmental obscurity to scrutiny. J. Hazard. Mater. 2006, 136, 1–19. [Google Scholar] [CrossRef]
- Witten, M.L.; Sheppard, P.R.; Witten, B.L. Tungsten toxicity. Chem. Interact. 2012, 196, 87–88. [Google Scholar] [CrossRef]
- Bednar, A.; Boyd, R.; Jones, W.; McGrath, C.; Johnson, D.; Chappell, M.; Ringelberg, D.B. Investigations of tungsten mobility in soil using column tests. Chemosphere 2009, 75, 1049–1056. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2007, 136, 227–238. [Google Scholar] [CrossRef]
- Huq, S.I.; Joardar, J.; Parvin, S.; Correll, R.; Naidu, R. Arsenic Contamination in Food-chain: Transfer of Arsenic into Food Materials through Groundwater Irrigation. J. Heal. Popul. Nutr. 2006, 24, 305–316. [Google Scholar]
- Islam, S.; Ahmed, K.; Mamun, H.A.; Eaton, D.W. Arsenic in the food chain and assessment of population health risks in Bangladesh. Environ. Syst. Decis. 2017, 37, 344–352. [Google Scholar] [CrossRef]
- Ficklin, W.H.; Plumlee, G.S.; Smith, K.S.; McHugh, J.B. Geochemical Classification of Mine Drainages and Natural Drainages in Mineralized Areas. In Proceedings of the 7th International Symposium on Water-Rock Interaction, Park City, UT, USA, 13–18 July 1992; pp. 381–384. [Google Scholar]
- Correia, V.F.; Favas, P.J.; Sá, A.; Lopes, F.; Andrade, A.I.; Henriques, M.H.; Quinta-Ferreira, M.; Dos Reis, R.P.; Barata, M.T. Impacto das drenagens ácidas das minas de Regoufe e Rio de Frades (Geoparque Arouca) na qualidade de água superficial. In Para Conhecer a Terra: Memórias e Notícias de Geociências no Espaço Lusófono; Coimbra University Press: Coimbra, Portugal, 2012; pp. 369–377. [Google Scholar]
- Plumlee, G.; Logsdon, M.; Filipek, L.; Smith, K.S.; Huyck, H.L.; Nordstrom, D.K.; Mills, A.; Alpers, C.; Ranville, J.; Schmiermund, R.; et al. The Environmental Geochemistry of Mineral Deposits. Environ. Geochem. Miner. Depos. 1997, 6, 133–160. [Google Scholar] [CrossRef]
- Cravotta, C.A. Dissolved metals and associated constituents in abandoned coal-mine discharges, Pennsylvania, USA. Part 2: Geochemical controls on constituent concentrations. Appl. Geochem. 2008, 23, 203–226. [Google Scholar] [CrossRef]
- Favas, P.J.C.; Pratas, J. Characterization of acid mine drainage at the Regoufe mine, Arouca Geopark, Northern Portugal. In Proceedings of the International Multidisciplinary Scientific GeoConference (SGEM), Vienna, Austria, 27–29 November 2017; International Multidisciplinary Scientific GeoConferences (SGEM): Sofia, Bulgaria, 2017; Volume 17, pp. 205–210. [Google Scholar] [CrossRef]
- DRE. Decreto-Lei nº 236/98, Diário da República; I Série-A. Technical Report No. 176; DRE: Lisboa, Portugal, 1998. [Google Scholar]
- DRE. Decreto-Lei nº 152/2017, Diário da República; I 1ª Série. Technical Report No. 235; DRE: Lisboa, Portugal, 2017. [Google Scholar]

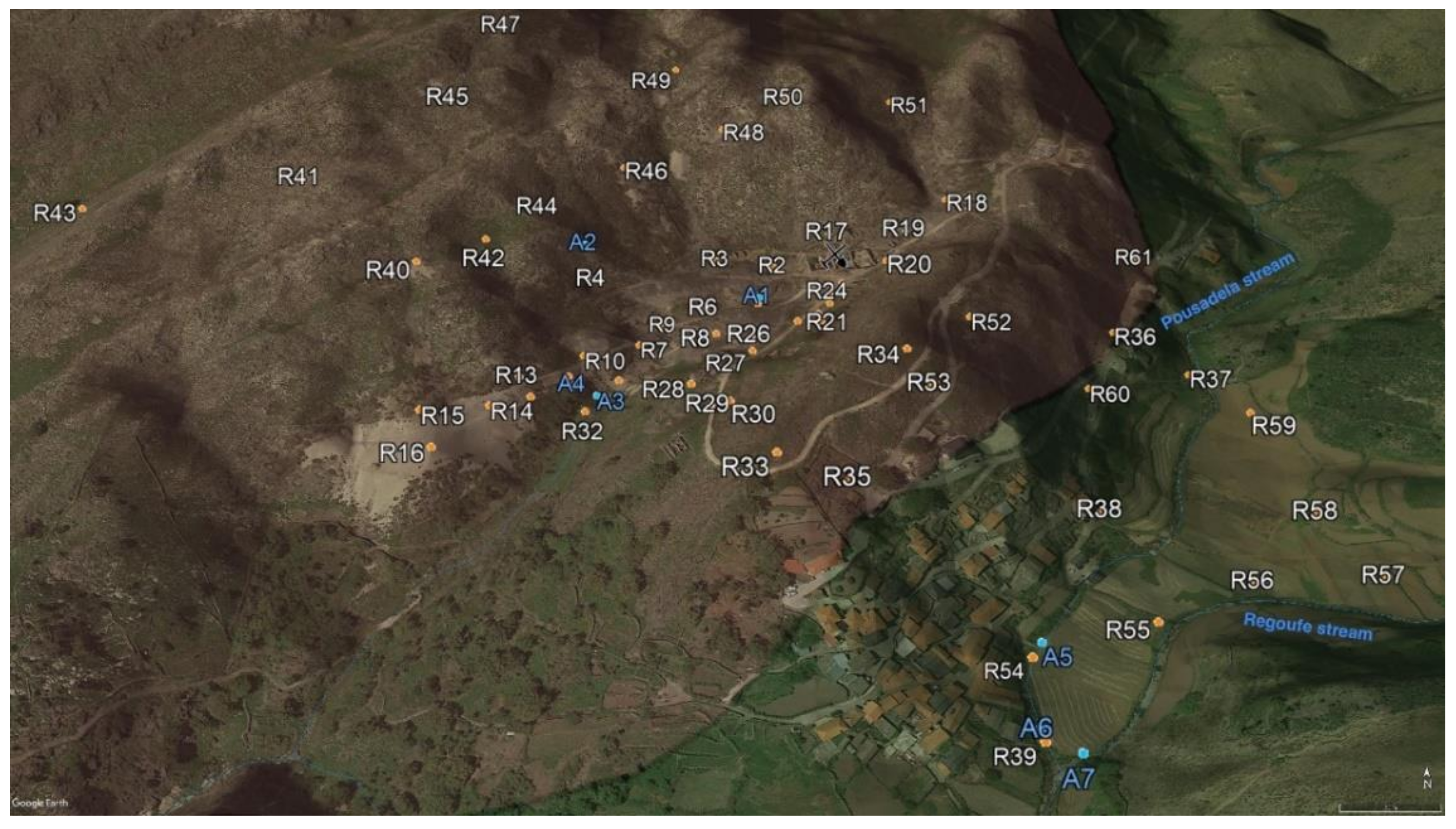
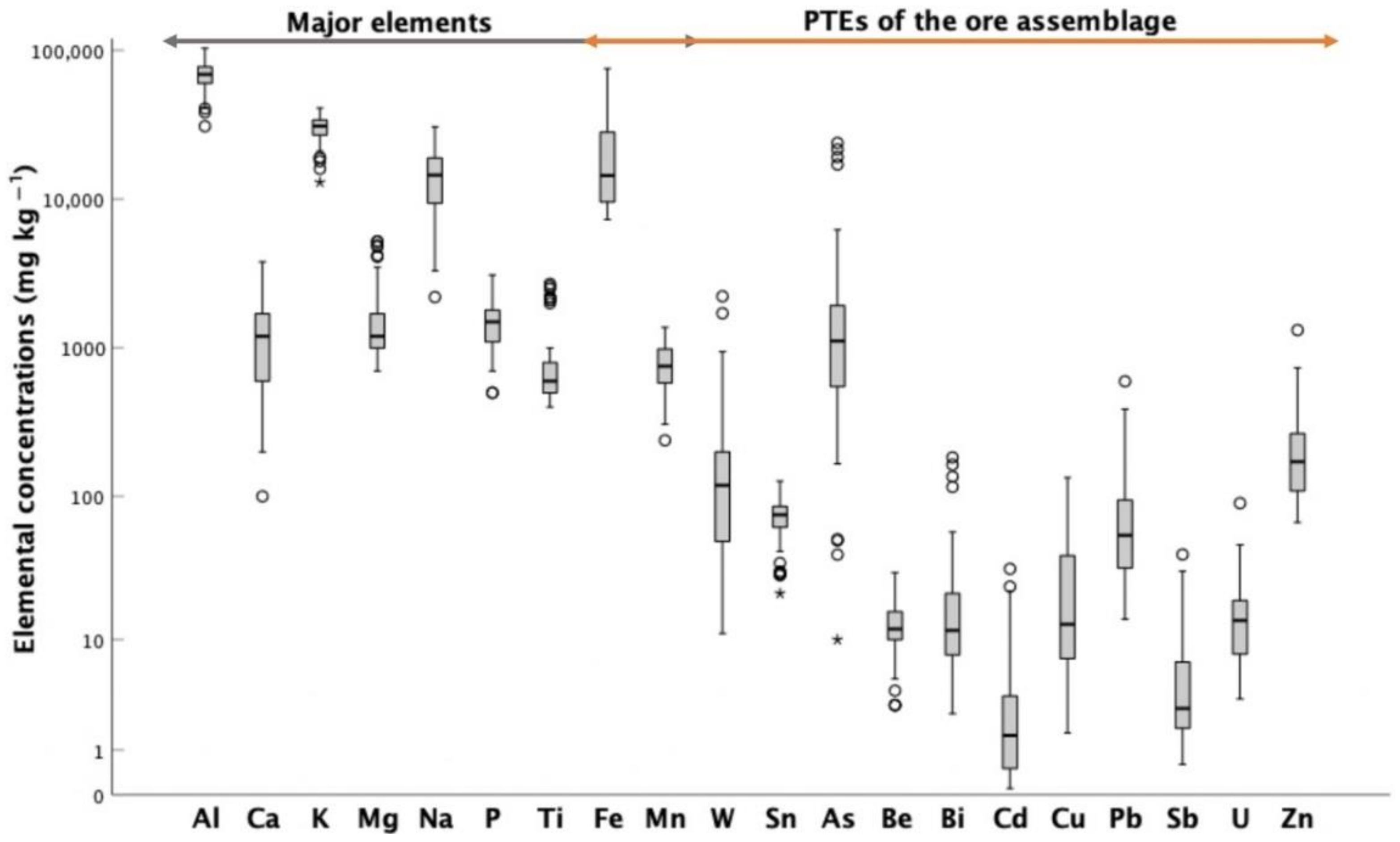
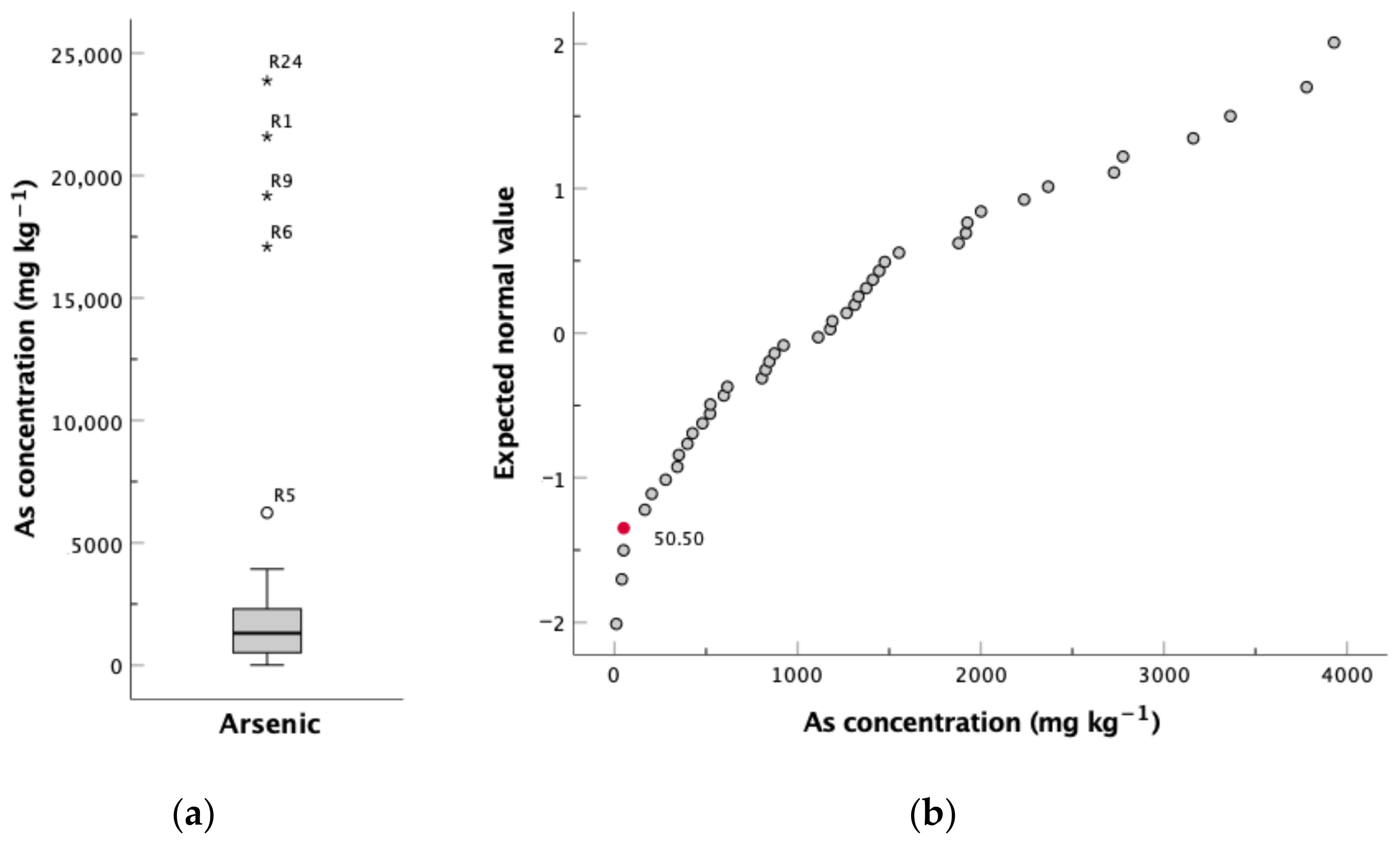
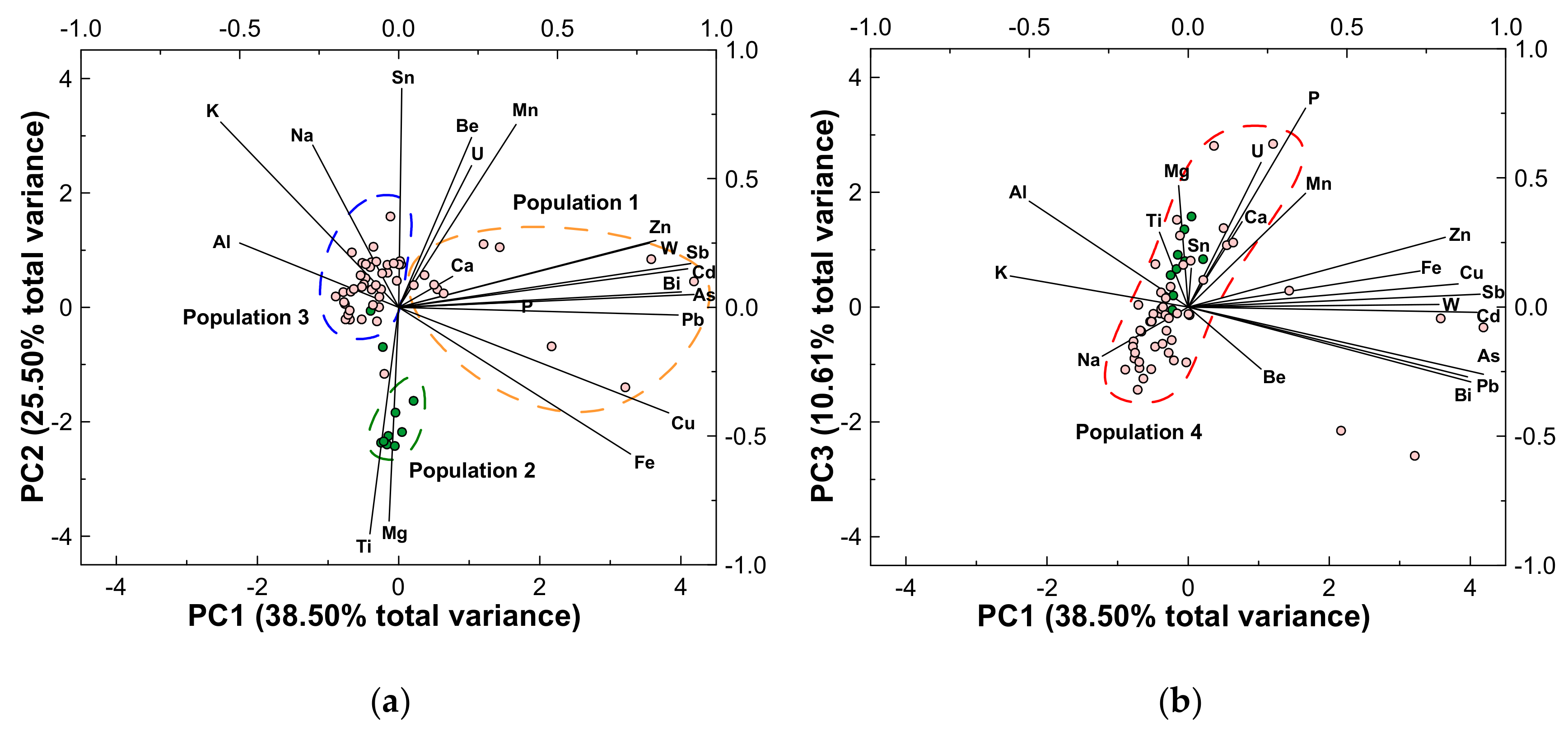
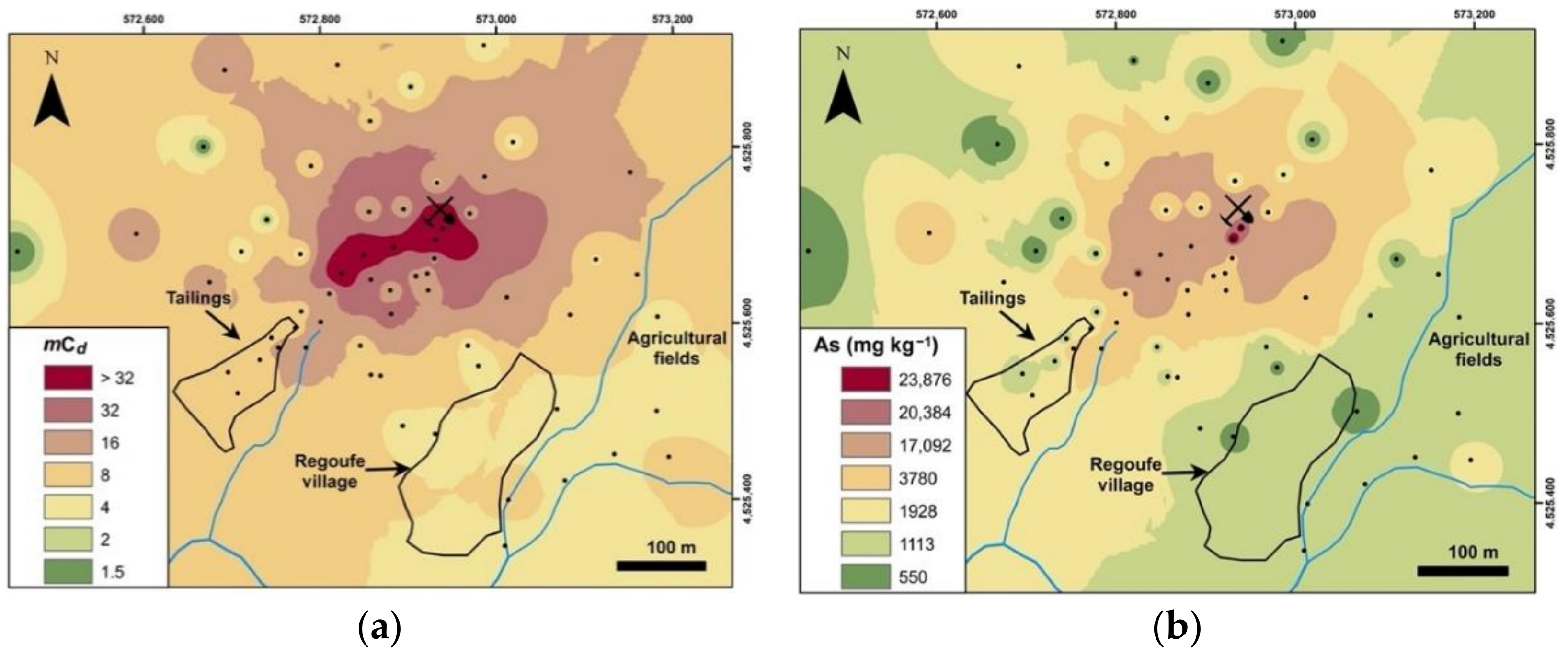
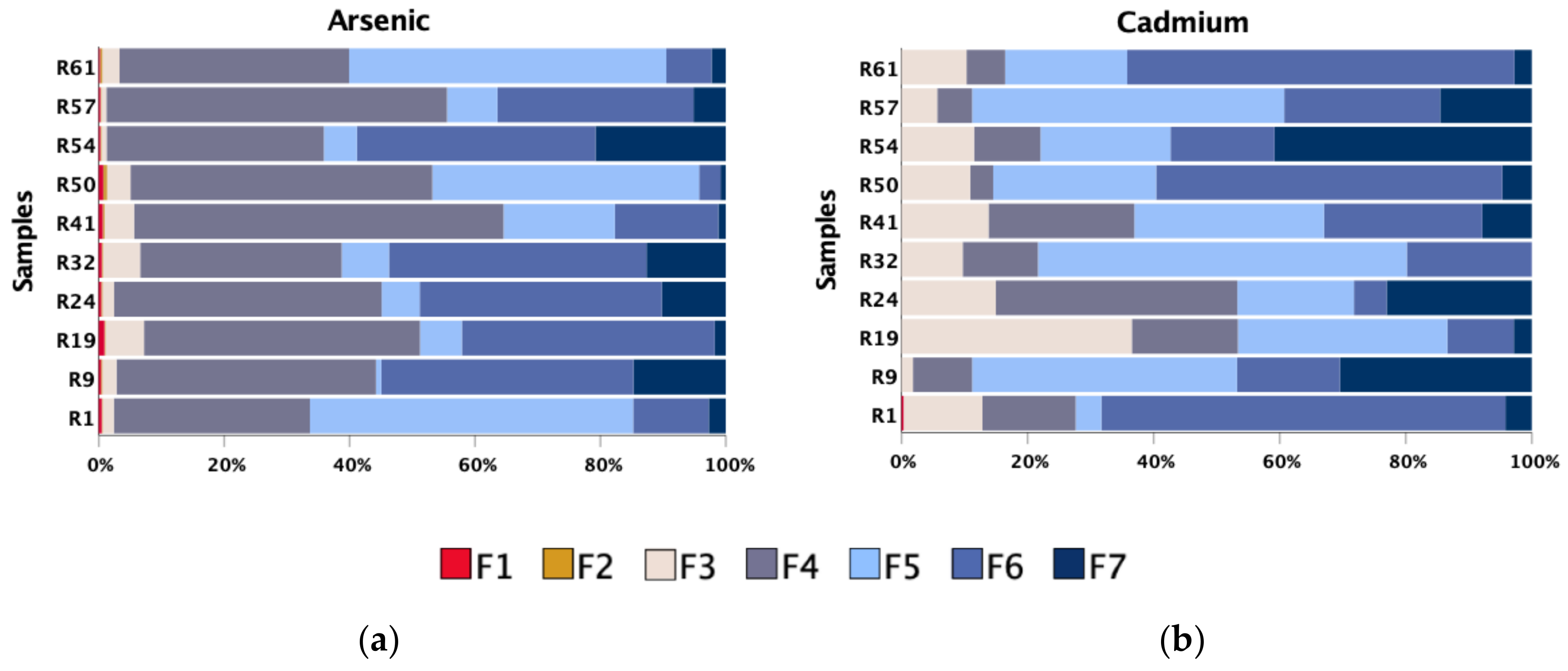
| Parameter | pHCaCl2 | EC | SOM | CEC | Fe | Mn | As | Bi | Cd | Cu | Pb | Sb | U | W | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | μS cm−1 | % | cmol+ kg−1 | % | mg kg−1 | ||||||||||
| Minimum | 3.40 | 7.80 | 0.14 | 0.16 | 0.73 | 239 | 10.2 | 2.50 | 0.06 | 1.6 | 14.1 | 0.64 | 3.40 | 11.1 | 66.5 |
| Median | 4.30 | 29.2 | 7.6 | 16 | 1.4 | 755 | 1113 | 11.7 | 1.5 | 13.0 | 54.4 | 2.8 | 13.8 | 119 | 172 |
| Maximum | 6.00 | 158 | 49 | 48 | 7.5 | 1374 | 23,876 | 183 | 32 | 134 | 597 | 40 | 89.9 | 2226 | 1320 |
| Mean | 4.34 | 43.2 | 9.5 | 17 | 2.1 | 787 | 2560 | 23.3 | 3.6 | 25.0 | 83.9 | 5.9 | 16.7 | 199 | 238 |
| SD | 0.48 | 35.5 | 8.7 | 12 | 1.6 | 281 | 4948 | 35.9 | 6.0 | 26.7 | 101 | 7.9 | 13.5 | 359 | 211 |
| GBlocal | - | - | - | - | 4.5 | 387 | 46.4 | 4.00 | 0.30 | 27.5 | 36.9 | 1.1 | 4.62 | 13.7 | 140 |
| GBgr | - | - | - | - | 0.89 | 530 | 50.5 | 4.00 | 0.20 | 4.2 | 24.7 | 1.5 | 5.90 | 23.1 | 86.8 |
| GBm 1 | - | - | - | - | 8.2 | 244 | 42.2 | N.A. | 0.39 | 50.9 | 49.2 | 0.69 | 3.34 | 4.2 | 193 |
| World 2 | - | - | - | - | 3.5 | 488 | 6.8 | 0.42 | 0.41 | 38.9 | 27.0 | 0.67 | 3.00 | 1.7 | 70.0 |
| Portugal 3 | - | - | - | - | 2.4 | 481 | 15.0 | 0.40 | 0.30 | 18.6 | 19.0 | 1.6 | 3.00 | 1.0 | 50.6 |
| Principal Component | PC1 | PC2 | PC3 |
|---|---|---|---|
| Eigenvalue | 7.00 | 5.10 | 2.12 |
| Proportion in total variance | 38.50 | 25.50 | 10.61 |
| Cumulative proportion | 38.50 | 63.99 | 74.60 |
| Eigenvector (loading) | |||
| Al | −0.50 | 0.25 | 0.41 |
| Ca | 0.17 | 0.12 | 0.33 |
| K | −0.56 | 0.72 | 0.12 |
| Mg | −0.03 | −0.83 | 0.47 |
| Na | −0.27 | 0.63 | −0.19 |
| P | 0.37 | 0.02 | 0.77 |
| Ti | −0.09 | −0.88 | 0.29 |
| Fe | 0.73 | −0.57 | 0.14 |
| Mn | 0.37 | 0.71 | 0.44 |
| W | 0.79 | 0.25 | 0.01 |
| Sn | 0.01 | 0.85 | 0.15 |
| As | 0.93 | 0.05 | −0.26 |
| Be | 0.23 | 0.66 | −0.24 |
| Bi | 0.89 | 0.06 | −0.29 |
| Cd | 0.91 | 0.15 | −0.02 |
| Cu | 0.85 | −0.41 | 0.09 |
| Pb | 0.88 | −0.03 | −0.27 |
| Sb | 0.92 | 0.17 | 0.05 |
| U | 0.23 | 0.55 | 0.56 |
| Zn | 0.81 | 0.26 | 0.27 |
| Samples (%) | mCd Value | Contamination Classes |
|---|---|---|
| 4.9 | mCd < 1.5 | None to very low |
| 1.6 | 1.5 ≤ mCd < 2 | Low |
| 26.2 | 2 ≤ mCd < 4 | Moderate |
| 29.5 | 4 ≤ mCd < 8 | High |
| 27.9 | 8 ≤ mCd < 16 | Very high |
| 1.6 | 16 ≤ mCd < 32 | Extremely high |
| 8.2 | 32 ≥ mCd | Ultra-high |
| Parameter | pH | EC | Cl | HCO3 | SO4 | Ca | K | Mg | Na | As | Cd | Mn | Pb | Sb | U | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | μS cm−1 | mg L−1 | μg L−1 | ||||||||||||||
| Wet season | Minimum | 5.99 | 19.2 | 3.7 | 5.92 | 1.0 | 1 | 0.7 | 0.2 | 0.2 | 0.78 | 0.04 | 1.04 | 0.21 | 0.01 | 0.001 | 19.6 |
| Median | 6.11 | 24.2 | 3.9 | 11.8 | 2.4 | 2.4 | 1 | 0.2 | 0.3 | 36.8 | 0.76 | 8.51 | 0.46 | 0.08 | 0.25 | 71.0 | |
| Maximum | 7.02 | 28.8 | 4.5 | 13.8 | 3.2 | 3.2 | 1.6 | 0.3 | 0.7 | 134 | 2.88 | 13.6 | 0.98 | 0.54 | 0.57 | 144 | |
| Mean | 6.25 | 24.4 | 4.0 | 10.7 | 2.2 | 2.2 | 1 | 0.2 | 0.4 | 49.9 | 1.25 | 8.08 | 0.51 | 0.14 | 0.24 | 72.6 | |
| SD | 0.35 | 3.48 | 0.27 | 2.51 | 0.78 | 0.78 | 0.3 | 0.05 | 0.24 | 58.0 | 1.13 | 4.87 | 0.27 | 0.19 | 0.24 | 47.9 | |
| Dry season | Minimum | 6.61 | 34.5 | 1.2 | 19.7 | 0.8 | 1.3 | 0.4 | 0.6 | 2.8 | 2.37 | 0.4 | 11.1 | 0.13 | 0.01 | 0.01 | 25.9 |
| Median | 6.66 | 46.0 | 4.3 | 19.7 | 4 | 1.9 | 0.8 | 0.7 | 3.9 | 16.8 | 0.75 | 12.5 | 0.24 | 0.02 | 0.01 | 40.8 | |
| Maximum | 6.88 | 51.5 | 4.7 | 19.7 | 4.6 | 2.6 | 0.9 | 1.2 | 3.9 | 206 | 9.45 | 12.5 | 0.51 | 0.36 | 0.64 | 484 | |
| Mean | 6.72 | 44.0 | 3.4 | 19.7 | 3.1 | 1.9 | 0.7 | 0.8 | 3.6 | 75.0 | 3.53 | 12.0 | 0.29 | 0.13 | 0.22 | 184 | |
| SD | 0.14 | 8.67 | 1.92 | 0 | 2.04 | 0.63 | 0.3 | 0.28 | 0.63 | 114 | 5.13 | 0.82 | 0.19 | 0.2 | 0.36 | 260 | |
| Guideline values | Drinking water 1 | 6.5–9.5 | 2500 | 250 | N.A. | 250 | 100 | 50 | 12 | 200 | 10 | 5.0 | 50 | 10 | 5.0 | N.A. | 3000 |
| Irrigation water 2 | 4.5–9.0 | 1000 | 70 | N.A. | 575 | N.A. | N.A. | N.A. | N.A. | 10,000 | 50 | 10,000 | 20,000 | N.A. | N.A. | 10,000 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durães, N.; Portela, L.; Sousa, S.; Patinha, C.; da Silva, E.F. Environmental Impact Assessment in the Former Mining Area of Regoufe (Arouca, Portugal): Contributions to Future Remediation Measures. Int. J. Environ. Res. Public Health 2021, 18, 1180. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18031180
Durães N, Portela L, Sousa S, Patinha C, da Silva EF. Environmental Impact Assessment in the Former Mining Area of Regoufe (Arouca, Portugal): Contributions to Future Remediation Measures. International Journal of Environmental Research and Public Health. 2021; 18(3):1180. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18031180
Chicago/Turabian StyleDurães, Nuno, Luís Portela, Sara Sousa, Carla Patinha, and Eduardo Ferreira da Silva. 2021. "Environmental Impact Assessment in the Former Mining Area of Regoufe (Arouca, Portugal): Contributions to Future Remediation Measures" International Journal of Environmental Research and Public Health 18, no. 3: 1180. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18031180







