First Insight into Nutraceutical Properties of Local Salento Cichorium intybus Varieties: NMR-Based Metabolomic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf Samples Collection
2.2. Sample Preparation for 1H NMR Analysis
2.3. 1H-NMR Spectra Acquisition and Processing
2.4. Multivariate Statistical Analysis
2.5. Chemicals
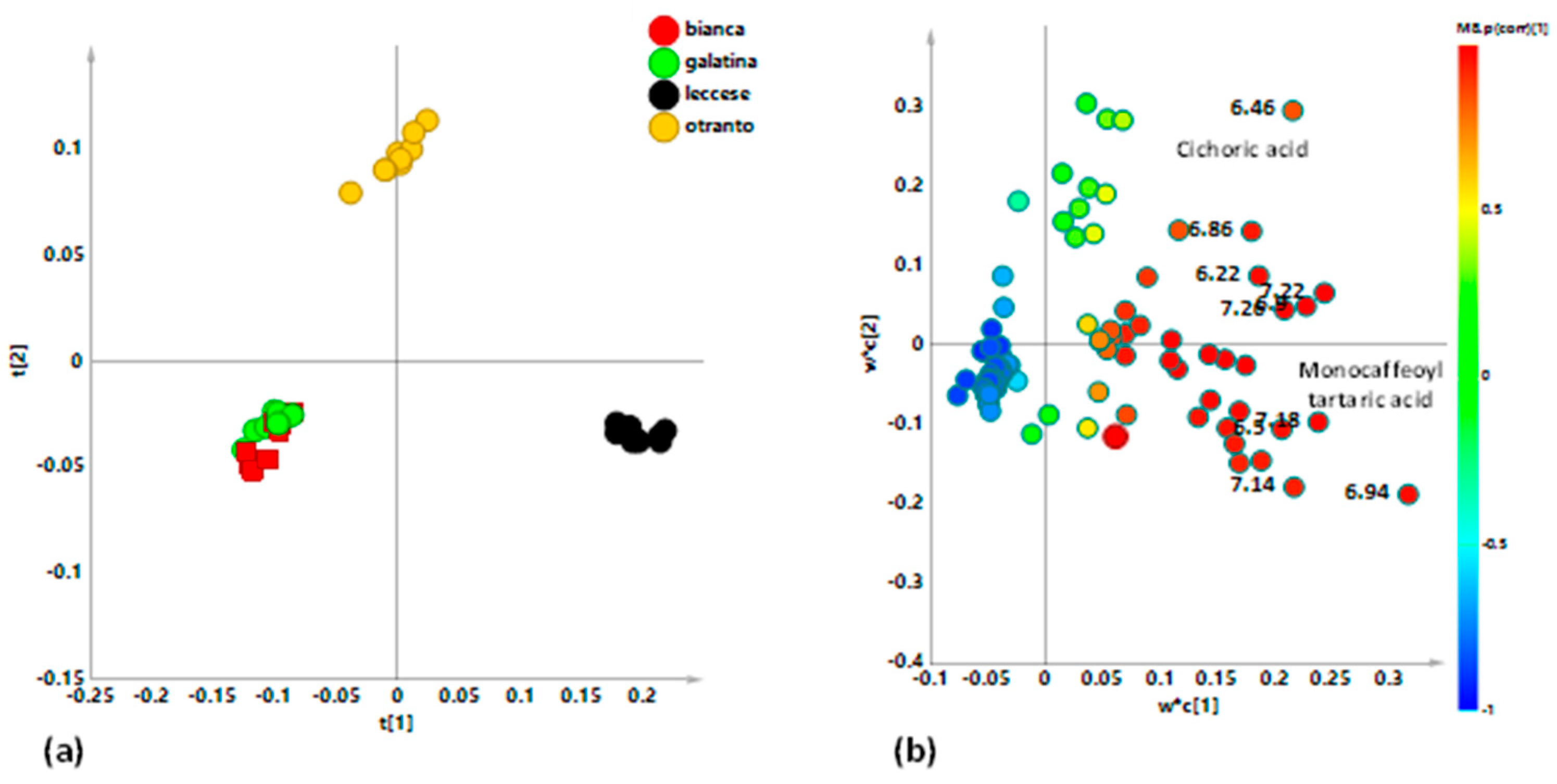
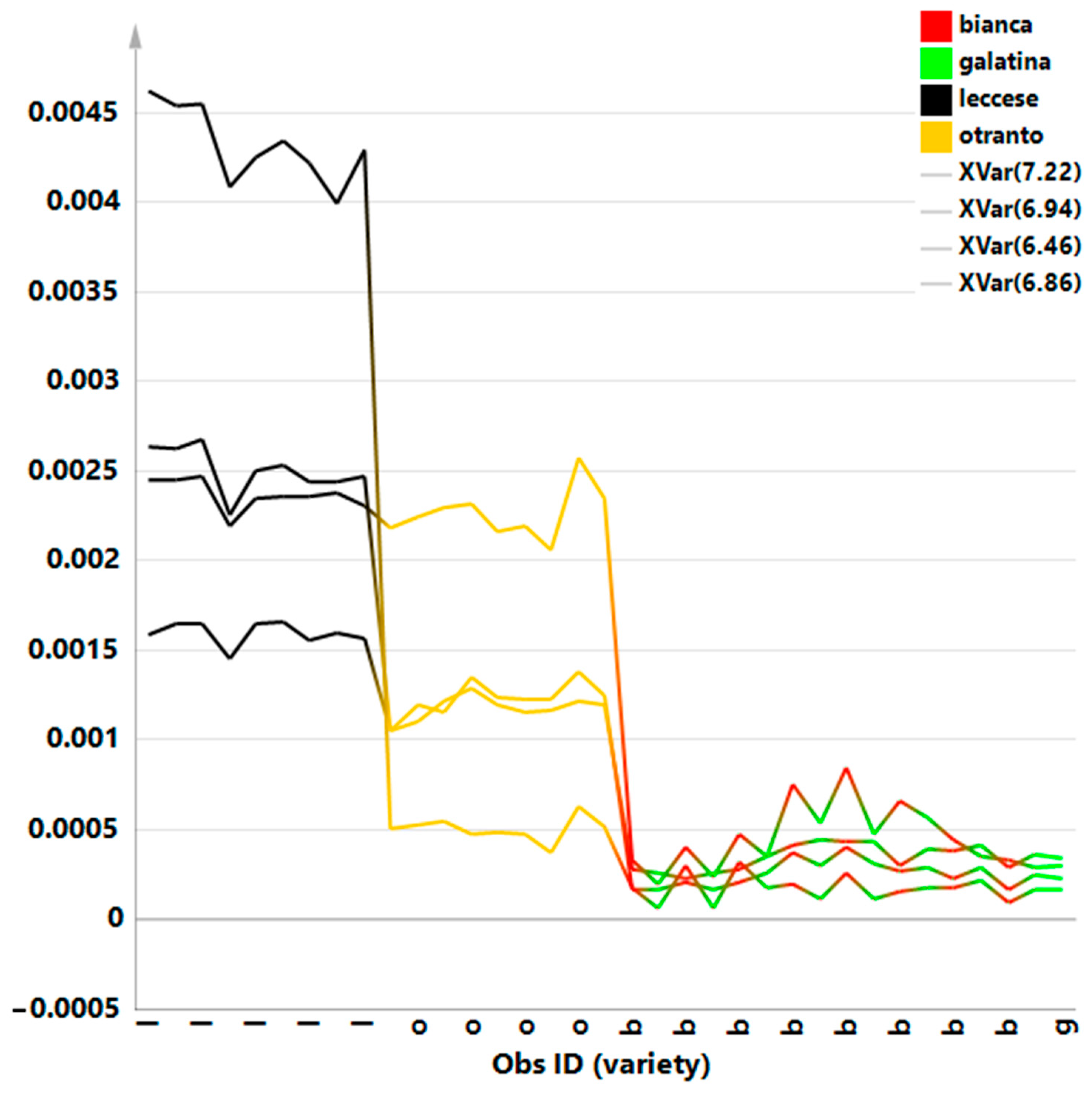
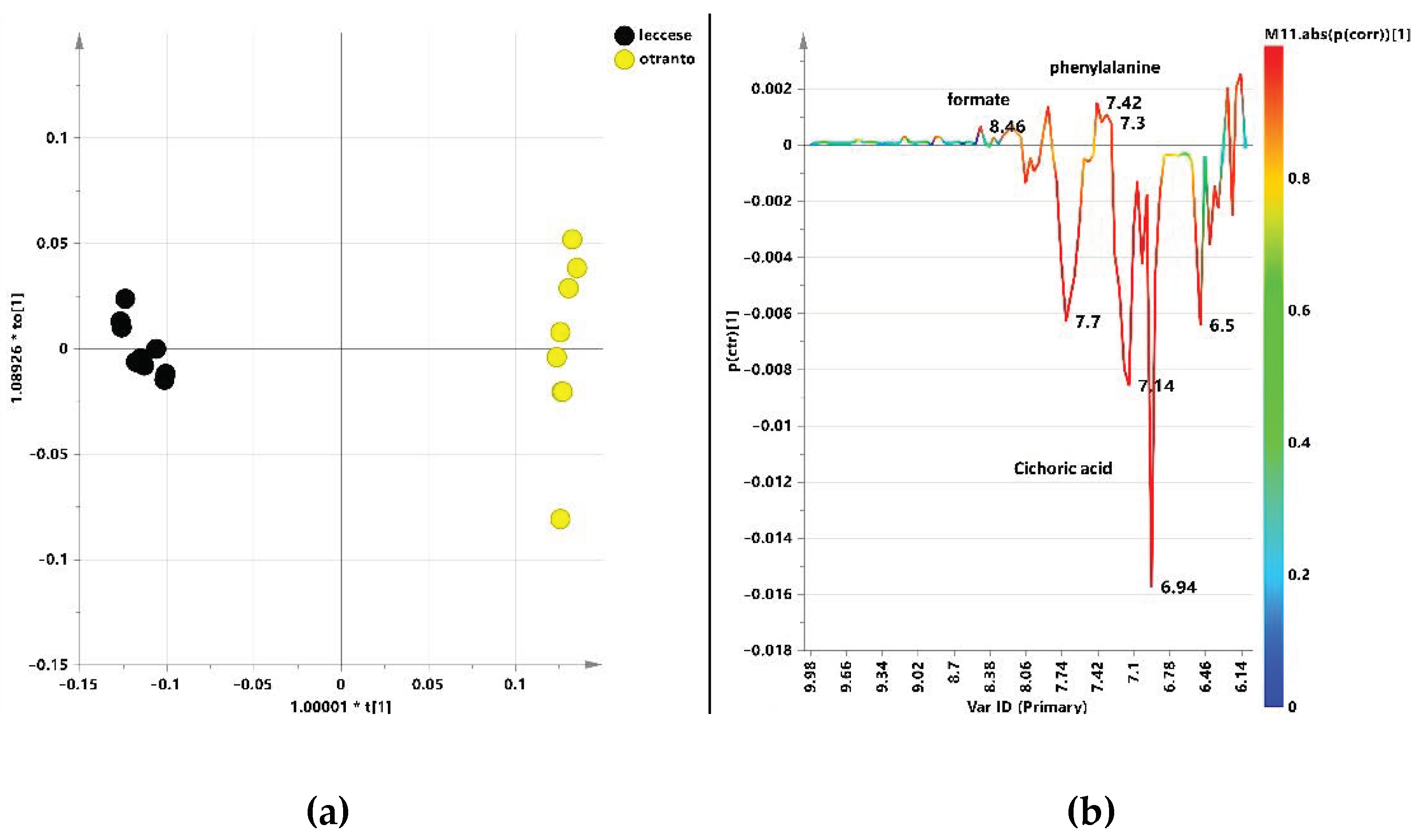
3. Results and Discussion
3.1. Visual Inspection of 1H NMR Spectra
3.2. Multivariate Statistical Analysis
3.3. Quantitative Metabolites Comparison
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diet, W. Nutrition and the Prevention of Chronic Diseases; Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series; WHO: Geneva, Switzerland, 2003; Volume 916, pp. 34–38. [Google Scholar]
- Sranacharoenpong, K.; Soret, S.; Harwatt, H.; Wien, M.; Sabate, J. The Environmental Cost of Protein Food Choices. Public Health Nutr. 2015, 18, 2096. [Google Scholar]
- Da Silva, B.V.; Barreira, J.C.; Oliveira, M.B.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Technol. 2016, 50, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Renna, M.; Montesano, F.F.; Signore, A.; Gonnella, M.; Santamaria, P. BiodiverSO: A Case Study of Integrated Project to Preserve the Biodiversity of Vegetable Crops in Puglia (Southern Italy). Agriculture 2018, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Accogli, R.; Conversa, G.; Luigi, R.; Gabriella, S.; Pietro, S. Nuovo Almanacco BiodiverSO-Biodiversità delle Specie Orticole della Puglia; Università degli Studi di Bari: Bari, Italy, 2018. [Google Scholar]
- Mosaddegh, M.; Naghibi, F.; Moazzeni, H.; Pirani, A.; Esmaeili, S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012, 141, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Ravishankar, G.A. Cichorium intybus L.–cultivation, processing, utility, value addition and biotechnology, with an emphasis on current status and future prospects. J. Sci. Food Agric. 2001, 81, 467–484. [Google Scholar] [CrossRef]
- Lucchin, M.V.S.; Barcaccia, G.; Parini, P. Chicory and endive. In Vegetables, I: Asteraceae, Brassicaceae, Chenopodiaceae, and Cucurbitaceae; Prohens-Tomás, G., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; Volume 426. [Google Scholar]
- Wang, Q.; Cui, J. Perspectives and utilization technologies of chicory (Cichorium intybus L.): A review. Afr. J. Biotechnol. 2011, 10, 1966–1977. [Google Scholar]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetto, M.; Lante, A.; Vanzani, P.; Spettoli, P.; Scarpa, M.; Rigo, A. Red Chicories as Potent Scavengers of Highly Reactive Radicals: A Study on Their Phenolic Composition and Peroxyl Radical Trapping Capacity and Efficiency. J. Agric. Food Chem. 2005, 53, 8169–8175. [Google Scholar] [CrossRef]
- Calabrese, N.; Pace, B.; Cantore, V.; Carito, A.; Damato, G. Epoche di raccolta, produzione e qualità di cicoria catalogna per la surgelazione e la IV gamma. Riv. Agron. 2009, 4, 559–564. [Google Scholar]
- Bianco, V.; Calabrese, N. Origine ed Evoluzione, Morfologia e Fisiologia; Script: Bologna, Italy, 2011; pp. 2–23. [Google Scholar]
- Al-Snafi, A.E. Medical importance of Cichorium intybus–A review. IOSR J. Pharm. 2016, 6, 41–56. [Google Scholar]
- Perović, J.; Šaponjac, V.T.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, F.; Manco, M.A.; D’Antuono, L.F. Variation of sesquiterpene lactones and phenolics in chicory and endive germplasm. J. Food Compos. Anal. 2015, 39, 77–86. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.-H.; Oh, Y.-T.; Chon, J.-W.; Kim, H.; Jeong, D.-K.; Kim, H.-S.; Kim, Y.-G.; Song, K.-Y.; Kim, Y.-J. Production of Bioactive Yoghurt containing Cichorium intybus L. (Chicory) Extract-Preliminary Study. J. Milk Sci. Biotechnol. 2017, 35, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Hellberg, R.S.; DeWitt, C.A.M.; Morrissey, M.T. Risk-Benefit Analysis of Seafood Consumption: A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 490–517. [Google Scholar] [CrossRef] [Green Version]
- Brennan, L. Metabolomics in nutrition research: Current status and perspectives. Biochem. Soc. Trans. 2013, 41, 670–673. [Google Scholar] [CrossRef]
- Savorani, F.; Rasmussen, M.A.; Mikkelsen, M.S.; Engelsen, S.B. A primer to nutritional metabolomics by NMR spectroscopy and chemometrics. Food Res. Int. 2013, 54, 1131–1145. [Google Scholar] [CrossRef] [Green Version]
- Spraul, M.; Schütz, B.; Humpfer, E.; Mörtter, M.; Schäfer, H.; Koswig, S.; Rinke, P. Mixture analysis by NMR as applied to fruit juice quality control. Magn. Reson. Chem. 2009, 47, S130–S137. [Google Scholar] [CrossRef]
- Carrieri, D.; McNeely, K.; De Roo, A.C.; Bennette, N.; Pelczer, I.; Dismukes, G.C. Identification and quantification of water-soluble metabolites by cryoprobe-assisted nuclear magnetic resonance spectroscopy applied to microbial fermentation. Magn. Reson. Chem. 2009, 47, S138–S146. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]
- Cubero-Leon, E.; Peñalver, R.; Maquet, A. Review on metabolomics for food authentication. Food Res. Int. 2014, 60, 95–107. [Google Scholar] [CrossRef]
- Gomez-Casati, D.F.; Zanor, M.I.; Busi, M.V. Metabolomics in Plants and Humans: Applications in the Prevention and Diagnosis of Diseases. BioMed Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- D.M. n. 1862 del 18/01/2018 Modalità di Funzionamento dell’Anagrafe Nazionale della Biodiversità di Interesse Agricolo e Alimentare; Mipaaf: Roma, Italy, 2018.
- Trisorio, A. Linee guida per la conservazione e la caratterizzazione della biodiversità vegetale, animale e microbica di interesse per l’agricoltura. In Piano Nazionale Sulla Biodiversità di Interesse Agricolo: Sintesi; INEA—Istituto Nazionale di Economia Agraria: Roma, Italy, 2013. [Google Scholar]
- Ritota, M.; Casciani, L.; Valentini, M. PGI chicory (Cichorium intybus L.) traceability by means of HRMAS-NMR spectroscopy: A preliminary study. J. Sci. Food Agric. 2012, 93, 1665–1672. [Google Scholar] [CrossRef]
- Wei, F.; Furihata, K.; Zhang, M.; Miyakawa, T.; Tanokura, M. Use of NMR-Based Metabolomics To Chemically Characterize the Roasting Process of Chicory Root. J. Agric. Food Chem. 2016, 64, 6459–6465. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Brosio, E.; Gianferri, R.; Segre, A. Metabolic profile of lettuce leaves by high-field NMR spectra. Magn. Reson. Chem. 2005, 43, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Berg, R.A.; Hoefsloot, H.C.J.; Westerhuis, J.A.; Smilde, A.K.; Van Der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kettaneh, N.; Berglund, A.; Wold, S. PCA and PLS with very large data sets. Comput. Stat. Data Anal. 2005, 48, 69–85. [Google Scholar] [CrossRef]
- Jackson, J.E. A User’s Guide to Principal Components; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 587. [Google Scholar]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. BioSyst. 2014, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Eriksson, L.; Trygg, J.; Kettaneh, N. The PLS Method—Partial Least Squares Projections to Latent Structures—And Its Applications in Industrial RDP (Research, Development, and Production); Umeå University: Umeå, Sweden, 2004. [Google Scholar]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Eriksson, L.; Byrne, T.; Johansson, E.; Trygg, J.; Vikström, C. Multi-and Megavariate Data Analysis Basic Principles and Applications; Umetrics Academy: Umeå, Sweden, 2013; Volume 1. [Google Scholar]
- Wheelock, Å.M.; Wheelock, C.E. Trials and tribulations of ‘omics data analysis: Assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. BioSyst. 2013, 9, 2589–2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girelli, C.R.; Accogli, R.; Del Coco, L.; Angilè, F.; De Bellis, L.; Fanizzi, F.P. 1H-NMR-based metabolomic profiles of different sweet melon (Cucumis melo L.) Salento varieties: Analysis and comparison. Food Res. Int. 2018, 114, 81–89. [Google Scholar] [CrossRef]
- Girelli, C.R.; De Pascali, S.A.; Del Coco, L.; Fanizzi, F.P. Metabolic profile comparison of fruit juice from certified sweet cherry trees (Prunus avium L.) of Ferrovia and Giorgia cultivars: A preliminary study. Food Res. Int. 2016, 90, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinkovič, L.; Hribar, J.; Vidrih, R.; Ilin, Ž.M.; Žnidarčič, D. Fatty acid composition of leaves of forced chicory (Cichorium intybus L.). Arch. Biol. Sci. 2015, 67, 647–653. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Carazzone, C.; Stauder, M.; Spratt, D.A.; Wilson, M.; Pratten, J.; Ciric, L.; Lingström, P.; Zaura, E. Identification of organic acids in Cichorium intybus inhibiting virulence-related properties of oral pathogenic bacteria. Food Chem. 2013, 138, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.; Moreira, E.M.; Jong, J.C.K.-D.; Darweesh, S.K.; Visser, T.; Voortman, T.; Bautista, P.K.; Chowdhury, R.; Gorman, D.; Bramer, W.M.; et al. Effects of choline on health across the life course: A systematic review. Nutr. Rev. 2015, 73, 500–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, T.C. A Comprehensive Review of Eggs, Choline, and Lutein on Cognition Across the Life-span. J. Am. Coll. Nutr. 2018, 37, 269–285. [Google Scholar] [CrossRef]
- Caudill, M.; da Costa, K.-A.; Zeisel, S.; Hornick, B. Elevating awareness and intake of choline: An essential nutrient for public health. Nutr. Today 2011, 46, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Pitkin, R.M.; Allen, L.; Bailey, L.; Bernfield, M. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin and Choline; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Zeisel, S.H.; Da Costa, K.-A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberfroid, M.B. Inulin-Type Fructans: Functional Food Ingredients. J. Nutr. 2007, 137, 2493S–2502S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.-L.; Dai, L.-H.; Wu, Y.-H.; Yu, X.-P.; Zhang, Y.-Y.; Guan, R.-F.; Liu, T.; Zhao, J. Evaluation of Hepatocyteprotective and Anti-hepatitis B Virus Properties of Cichoric Acid from Cichorium intybus Leaves in Cell Culture. Biol. Pharm. Bull. 2014, 37, 1214–1220. [Google Scholar] [CrossRef] [Green Version]
- Tappy, L.; Lê, K.-A. Metabolic Effects of Fructose and the Worldwide Increase in Obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Jurgoński, A.; Milala, J.; Juśkiewicz, J.; Zduńczyk, Z.; Król, B. Composition of chicory root, peel, seed and leaf ethanol extracts and biological properties of their non-inulin fractions. Food Technol. Biotechnol. 2011, 49, 40–47. [Google Scholar]
- Wilson, R.G.; Smith, J.A.; Yonts, C.D. Chicory root yield and carbohydrate composition is influenced by cultivar selection, planting, and harvest date. Crop Sci. 2004, 44, 748–752. [Google Scholar] [CrossRef]
- Tarkowski, Ł.P.; Ende, W.V.D. Cold tolerance triggered by soluble sugars: A multifaceted countermeasure. Front. Plant Sci. 2015, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Bogdanović, J.; Mojović, M.; Milosavić, N.; Mitrović, A.; Vučinić, Ž.; Spasojević, I. Role of fructose in the adaptation of plants to cold-induced oxidative stress. Eur. Biophys. J. 2008, 37, 1241–1246. [Google Scholar] [CrossRef]
- De, L.; De, T. Healthy food for healthy life. J. Glob. Biosci. 2019, 8, 6453–6468. [Google Scholar]
- Gu, W.; Li, J. Chicory seeds: A potential source of nutrition for food and feed. J. Anim. Plant Sci. 2012, 13, 1736–1746. [Google Scholar]
- Druart, N.; Goupil, P.; Dewaele, E.; Boutin, J.; Rambour, S. Nitrate assimilation in chicory roots (Cichorium intybus L.) which acquire radial growth. J. Exp. Bot. 2000, 51, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Ghamarian, A.; Abdollahi, M.; Su, X.; Amiri, A.; Ahadi, A.; Nowrouzi, A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU J. Pharm. Sci. 2012, 20, 56. [Google Scholar] [CrossRef] [Green Version]
- Heimler, D.; Isolani, L.; Vignolini, P.; Romani, A. Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chem. 2009, 114, 765–770. [Google Scholar] [CrossRef]
- Khalaf, H.A.; El-Saadani, R.M.; El-Desouky, A.I.; Abdeldaiem, M.H.; Elmehy, M.E. Antioxidant and antimicrobial activity of gamma-irradiated chicory (Cichorium intybus L.) leaves and roots. J. Food Meas. Charact. 2018, 12, 1843–1851. [Google Scholar] [CrossRef]
- Schmidt, B.M.; Ilic, N.; Poulev, A.; Raskin, I. Toxicological evaluation of a chicory root extract. Food Chem. Toxicol. 2007, 45, 1131–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innocenti, M.; Gallori, S.; Giaccherini, C.; Ieri, F.; Vincieri, F.F.; Mulinacci, N. Evaluation of the Phenolic Content in the Aerial Parts of Different Varieties of Cichorium intybus L. J. Agric. Food Chem. 2005, 53, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Bahri, M.; Hance, P.; Grec, S.; Quillet, M.-C.; Trotin, F.; Hilbert, J.-L.; Hendriks, T. A “Novel” Protocol for the Analysis of Hydroxycinnamic Acids in Leaf Tissue of Chicory (Cichorium intybus L., Asteraceae). Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Wang, J.; Yuan, L.; Xiao, C.; Wang, Y.; Liu, X. Chicoric Acid Induces Apoptosis in 3T3-L1 Preadipocytes through ROS-Mediated PI3K/Akt and MAPK Signaling Pathways. J. Agric. Food Chem. 2013, 61, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Huang, F.; Xiang, X.; Fan, M.; Chen, T. Evaluation of the potential of chicoric acid as a natural food antioxidant. Exp. Ther. Med. 2018, 16, 3651–3657. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.-L.; Kao, C.-L.; Hung, C.-H.; Cheng, Y.-H.; Lin, H.-C.; Chu, P.-M. Chicoric acid is a potent anti-atherosclerotic ingredient by anti-oxidant action and anti-inflammation capacity. Oncotarget 2017, 8, 29600–29612. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M.-J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Wang, Y.; Diao, Z.; Li, J.; Ren, B.; Zhu, D.; Liu, Q.; Liu, Z.; Liu, X. Chicoric acid supplementation ameliorates cognitive impairment induced by oxidative stress via promotion of antioxidant defense system. RSC Adv. 2017, 7, 36149–36162. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Huang, G.; Gao, M.; Shen, X.; Gong, W.; Xu, Z.; Zeng, Y.; He, F. Chicoric acid suppresses BAFF expression in B lymphocytes by inhibiting NF-κB activity. Int. Immunopharmacol. 2017, 44, 211–215. [Google Scholar] [CrossRef]
- Crosby, D.C.; Lei, X.; Gibbs, C.G.; McDougall, B.R.; Robinson, W.E., Jr.; Reinecke, M.G. Design, synthesis, and biological evaluation of novel hybrid dicaffeoyltartaric/diketo acid and tetrazole-substituted L-chicoric acid analogue inhibitors of human immunodeficiency virus type 1 integrase. J. Med. Chem. 2010, 53, 8161–8175. [Google Scholar] [CrossRef]
- Reinke, R.A.; King, P.J.; Victoria, J.G.; McDougall, B.R.; Ma, G.; Mao, Y.; Reinecke, M.G.; Robinson, W.E. Dicaffeoyltartaric Acid Analogues Inhibit Human Immunodeficiency Virus Type 1 (HIV-1) Integrase and HIV-1 Replication at Nontoxic Concentrations. J. Med. Chem. 2002, 45, 3669–3683. [Google Scholar] [CrossRef]
- Reinke, R.A.; Lee, D.J.; McDougall, B.R.; King, P.J.; Victoria, J.; Mao, Y.; Lei, X.; Reinecke, M.G.; Robinson, W.E., Jr. L-chicoric acid inhibits human immunodeficiency virus type 1 integration in vivo and is a noncompetitive but reversible inhibitor of HIV-1 integrase in vitro. Virology 2004, 326, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Zhang, N.; Zhou, X.; Zhang, M.; Liu, Z.; Liu, X. Cichoric acid regulates the hepatic glucose homeostasis via AMPK pathway and activates the antioxidant response in high glucose-induced hepatocyte injury. RSC Adv. 2017, 7, 1363–1375. [Google Scholar] [CrossRef] [Green Version]
- Dalar, A.; Konczak, I. Cichorium intybus from Eastern Anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. Ind. Crop. Prod. 2014, 60, 79–85. [Google Scholar] [CrossRef]
- Kour, K.; Bani, S. Chicoric acid regulates behavioral and biochemical alterations induced by chronic stress in experimental Swiss albino mice. Pharmacol. Biochem. Behav. 2011, 99, 342–348. [Google Scholar] [CrossRef]
- Chan, L.; Zhou, S. Trigonelline: A Plant Alkaloid with Therapeutic Potential for Diabetes and Central Nervous System Disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef]






| Metabolites | Chemical Shifts δ (ppm) |
|---|---|
| Aminoacids | |
| Leucine | 0.97 (d, β-CH3), 1.72 (d, β-CH2) |
| Valine | 0.98 (d, CH3), 1.03 (d, CH3), 2.26 (m, β-CH) |
| Isoleucine | 1.00 (d, β−CH3) |
| Threonine | 1.32 (d, γ-CH3), 4.26 (α-CH) |
| Alanine | 1.47 (d, CH3), 3.79 (m, α-CH) |
| GABA (γ-aminobutyric acid) | 1.90 (m, β-CH2), 2.35 (t, α-CH2), 3.02 (t, γ-CH2) |
| Glutamine | 2.13 (m, β-CH2), 2.44 (m, γ-CH2), 3.76 (m, α-CH) |
| Glutamate | 2.36 (m, γ-CH2) |
| Asparagine | 2.95 (dd, β-CH2) |
| Tyrosine | 6.91 (m, CH3, H5), 7.19 (m, CH2, CH6) |
| Phenylalanine | 7.43 (m, CH-3, 5, ring), 7.37 (m, CH-4, ring),
7.30 (m, CH-2,6) |
| Sugars | |
| β-D-glucose | 3.26 (dd, CH-2), 3.48 (t, CH-3),4.64 (d, CH-1) |
| α-D-glucose | 3.5 (dd, H2), 5.23 (d, H1) |
| Sucrose | 3.55 (dd, CH-2), 3.67 (s, CH-2′), 3.81 (m, CH2-6,6′), 4.20 (d, H3′), 5.40 (d, CH-1) |
| α-D-fructofuranose | 4.01 (CH-5), 4.1 (d, CH-3,) |
| β-D-fructofuranose | 4.12 (m, CH-3, CH-4), 3.80 (m, CH-5) |
| β-D-fructopyranose | 4.02 (CH-5), 3.70, 3.56 (CH2-1,1′) |
| Inulin | 5.42 (m, CH-1), 4.28 (m, CH-3′) |
| Organic acids | |
| Malate | 2.39 (β-CH), 2.69 (β’-CH), 4.31 (α-CH) |
| Tartrate | 4.31(s, CH) |
| Fumarate | 6.52 (α, β-CH=CH) |
| Formate | 8.46 (s, HCOOH) |
| Phenolic compounds | |
| Cichoric acid | 5.54 (s, CH(O)COOH), 6.50 (d, =CH-COO−), 6.97 (d, CH5′), 7.26 (d, CH-2′), 7.72 (d, -CH=) |
| Monocaffeoyl tartaric acid | 6.89 (d, CH-5′), 7.62 (d, -CH=), 6.43 (d, =CH-COO−), 5.30 (d, CH(O)COOH) |
| Chlorogenic acid | 7.61 (d, -CH=), 6.35 (d, =CH-COO−), 5.32 (d, CH(O)COOH) |
| Other compounds | |
| fatty acids | 0.9 |
| uridine | 7.9 (d, CH-5, ring), 5.9 (CH-6, ring) |
| deoxyadenosine | 8.3 (s, CH-13 ring), 8.2(s, CH-11, ring) |
| trigonelline | 9.13 (s, CH-2), 8,84 (t, CH-3,5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girelli, C.R.; Serio, F.; Accogli, R.; Angilè, F.; De Donno, A.; Fanizzi, F.P. First Insight into Nutraceutical Properties of Local Salento Cichorium intybus Varieties: NMR-Based Metabolomic Approach. Int. J. Environ. Res. Public Health 2021, 18, 4057. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18084057
Girelli CR, Serio F, Accogli R, Angilè F, De Donno A, Fanizzi FP. First Insight into Nutraceutical Properties of Local Salento Cichorium intybus Varieties: NMR-Based Metabolomic Approach. International Journal of Environmental Research and Public Health. 2021; 18(8):4057. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18084057
Chicago/Turabian StyleGirelli, Chiara Roberta, Francesca Serio, Rita Accogli, Federica Angilè, Antonella De Donno, and Francesco Paolo Fanizzi. 2021. "First Insight into Nutraceutical Properties of Local Salento Cichorium intybus Varieties: NMR-Based Metabolomic Approach" International Journal of Environmental Research and Public Health 18, no. 8: 4057. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18084057







