Pea Protein-Derived Peptides Inhibit Hepatic Glucose Production via the Gluconeogenic Signaling in the AML-12 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Profiling of PPH
2.2. Cell Culture
2.3. Cell Viability
2.4. HGP Assay in AML-12 Cells
2.5. Western Blotting
2.6. qRT-PCT
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Peptide Profile
3.2. PPH Did Not Show Cytotoxicity in the AML-12 Cells
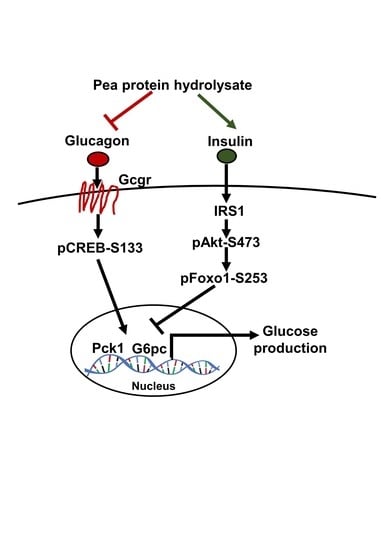
3.3. PPH Suppressed Glucagon-Induced HGP in the AML-12 Cells
3.4. PPH Regulated Glucagon Signaling in the AML-12 Cells
3.5. PPH-Activated Insulin Signaling in the AML-12 Cells
3.6. PPH Suppressed the Glucose Production in AML-12 Cells via the Glucagon Signaling but Not the Insulin Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Gromada, J.; Duttaroy, A.; Rorsman, P. The Insulin Receptor Talks to Glucagon? Cell Metab. 2009, 9, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, D.S.; Cherrington, A.D. Glucagon as a Critical Factor in the Pathology of Diabetes. Diabetes 2011, 60, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Accili, D.; Arden, K.C. FoxOs at the Crossroads of Cellular Metabolism, Differentiation, and Transformation. Cell 2004, 117, 421–426. [Google Scholar] [CrossRef]
- Rines, A.K.; Sharabi, K.; Tavares, C.D.J.; Puigserver, P. Targeting hepatic glucose metabolism in the treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2016, 15, 786–804. [Google Scholar] [CrossRef] [PubMed]
- Ismail-Beigi, F. Glycemic Management of Type 2 Diabetes Mellitus. N. Engl. J. Med. 2012, 366, 1319–1327. [Google Scholar] [CrossRef]
- Khan, M.I.; Anjum, F.M.; Sohaib, M.; Sameen, A. Tackling metabolic syndrome by functional foods. Rev. Endocr. Metab. Disord. 2013, 14, 287–297. [Google Scholar] [CrossRef]
- Cao, X.; Liao, W.; Wang, S. Food protein-derived bioactive peptides for the management of nutrition related chronic diseases. Adv. Food Nutr. Res. 2022, 101, 277–307. [Google Scholar]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re)formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Geerts, M.E.J.; Mienis, E.; Nikiforidis, C.V.; van der Padt, A.; van der Goot, A.J. Mildly refined fractions of yellow peas show rich behaviour in thickened oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2017, 41, 251–258. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Crombag, J.J.R.; Senden, J.M.G.; Waterval, W.A.H.; Bierau, J.; Verdijk, L.B.; van Loon, L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids 2018, 50, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate composition and anti-nutritional factors of fava-bean (Vicia faba), green-pea and yellow-pea (Pisum sativum) flour. J. Food Compos. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- López-Barrios, L.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bioactive Peptides and Hydrolysates from Pulses and Their Potential Use as Functional Ingredients. J. Food Sci. 2014, 79, R273–R283. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, physicochemical properties of pea protein and its application in functional foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liang, Y.; Ye, X.; Zhang, H.; Lv, J.; Dong, H.; Lin, F.; Wen, X. Serum proteomic analysis reveals possible mechanism underlying physiological hemostasis of swing bladder. J. Proteom. 2022, 266, 104668. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-P.; Qian, Y.-F.; Qin, G.-Y.-X.; Zhao, L.-Y.; Chen, G.-T. Antidiabetic activities of glycoprotein from pea (Pisum sativum L.) in STZ-induced diabetic mice. Food Funct. 2021, 12, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, K.W.; Jeong, J.; Park, K.; Ryu, Y.; Moyo, K.M.; Kim, H.K.; Go, G.-W. Red Pepper (Capsicum annuum L.) Seed Extract Decreased Hepatic Gluconeogenesis and Increased Muscle Glucose Uptake In Vitro. J. Med. Food 2018, 21, 665–671. [Google Scholar] [CrossRef]
- Liao, W.; Fan, H.; Wu, J. Identification of angiotensin converting enzyme 2 (ACE2) up-regulating peptides from pea protein hydrolysate. J. Funct. Foods 2019, 60, 103395. [Google Scholar] [CrossRef]
- Kornet, C.; Venema, P.; Nijsse, J.; van der Linden, E.; van der Goot, A.J.; Meinders, M. Yellow pea aqueous fractionation increases the specific volume fraction and viscosity of its dispersions. Food Hydrocoll. 2020, 99, 105332. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.-X.; Corke, H.; Gul, K.; Gan, R.-Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef]
- Kumari, T.; Deka, S.C. Potential health benefits of garden pea seeds and pods: A review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive Peptides in Cereals and Legumes: Agronomical, Biochemical and Clinical Aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef]
- Ruiz, R.; Olías, R.; Clemente, A.; Rubio, L.A. A Pea (Pisum sativum L.) Seed Vicilins Hydrolysate Exhibits PPARγ Ligand Activity and Modulates Adipocyte Differentiation in a 3T3-L1 Cell Culture Model. Foods 2020, 9, 793. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, R.; Fang, L.; Qin, X.; Cai, M.; Gu, R.; Lu, J.; Wang, Y. Hypoglycemic effects and biochemical mechanisms of Pea oligopeptide on high-fat diet and streptozotocin-induced diabetic mice. J. Food Biochem. 2019, 43, e13055. [Google Scholar] [CrossRef]
- Thondre, P.S.; Achebe, I.; Sampson, A.; Maher, T.; Guérin-Deremaux, L.; Lefranc-Millot, C.; Ahlström, E.; Lightowler, H. Co-ingestion of NUTRALYS® pea protein and a high-carbohydrate beverage influences the glycaemic, insulinaemic, glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) responses: Preliminary results of a randomised controlled trial. Eur. J. Nutr. 2021, 60, 3085–3093. [Google Scholar]
- Chen, Y.; Liu, H.; Wang, Y.; Yang, S.; Yu, M.; Jiang, T.; Lv, Z. Glycosaminoglycan from Apostichopus japonicus inhibits hepatic glucose production via activating Akt/FoxO1 and inhibiting PKA/CREB signaling pathways in insulin resistant hepatocytes. Food Funct. 2019, 10, 7565–7575. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Shen, J.Z.; Pan, Q.; Yang, W.; Yan, H.; Liu, H.; Ai, W.; Liao, W.; Guo, S. Epigallocatechin Gallate Inhibits Hepatic Glucose Production in Primary Hepatocytes via Downregulating PKA Signaling Pathways and Transcriptional Factor FoxO1. J. Agric. Food Chem. 2019, 67, 3651–3661. [Google Scholar] [CrossRef]
- Claessens, M.; Calame, W.; Siemensma, A.D.; van Baak, M.A.; Saris, W.H.M. The effect of different protein hydrolysate/carbohydrate mixtures on postprandial glucagon and insulin responses in healthy subjects. Eur. J. Clin. Nutr. 2009, 63, 48–56. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure–Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Wang, X.; Bhullar, K.S.; Fan, H.; Liao, W.; Qiao, Y.; Su, D.; Wu, J. Regulatory Effects of a Pea-Derived Peptide Leu-Arg-Trp (LRW) on Dysfunction of Rat Aortic Vascular Smooth Muscle Cells against Angiotensin II Stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef]
- Arora, H.; Shang, N.; Bhullar, K.S.; Wu, J. Pea protein-derived tripeptide LRW shows osteoblastic activity on MC3T3-E1 cells via the activation of the Akt/Runx2 pathway. Food Funct. 2020, 11, 7197–7207. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.; Cao, X.; Xia, H.; Wang, S.; Sun, G. Pea Protein-Derived Peptides Inhibit Hepatic Glucose Production via the Gluconeogenic Signaling in the AML-12 Cells. Int. J. Environ. Res. Public Health 2022, 19, 10254. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191610254
Liao W, Cao X, Xia H, Wang S, Sun G. Pea Protein-Derived Peptides Inhibit Hepatic Glucose Production via the Gluconeogenic Signaling in the AML-12 Cells. International Journal of Environmental Research and Public Health. 2022; 19(16):10254. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191610254
Chicago/Turabian StyleLiao, Wang, Xinyi Cao, Hui Xia, Shaokang Wang, and Guiju Sun. 2022. "Pea Protein-Derived Peptides Inhibit Hepatic Glucose Production via the Gluconeogenic Signaling in the AML-12 Cells" International Journal of Environmental Research and Public Health 19, no. 16: 10254. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph191610254