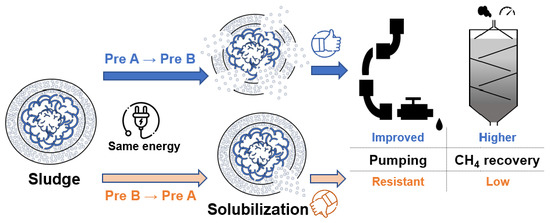
Series of Combined Pretreatment Can Affect the Solubilization of Waste-Activated Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
2.2. Pretreatment and BMP Test
2.3. Calculation and Analysis
3. Results and Discussion
3.1. Solubilization
3.2. EEM and MWD
3.3. CH4 Yield
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Term: | Abbreviation: |
| Waste-activated sludge | WAS |
| Combined pretreatment | CP |
| Disintegration degree | DD |
| Alkaline followed by thermal pretreatment | A→T |
| Thermal followed by alkaline pretreatment | T→A |
| Thermal followed by ultrasonic pretreatment | T→U |
| Ultrasonic followed by thermal pretreatment | U→T |
| Ultrasonic followed by alkaline pretreatment | U→A |
| Alkaline followed by ultrasonic pretreatment | A→U |
| Soluble microbial products | SMP |
| Anaerobic digestion | AD |
| Volatile solids | VS |
| Volatile suspended solids | VSS |
| Total solids | TS |
| Total chemical oxygen demand | TCOD |
| Soluble chemical oxygen demand | SCOD |
| Excitation-emission matrix | EEM |
| Molecular weight distribution | MWD |
| Biochemical methane potential | BMP |
| CH4 yield from total fraction of pretreated waste-activated sludge | MYt |
| CH4 yield from soluble fraction of pretreated waste-activated sludge | MYs |
| CH4 yield from particulate fraction of pretreated waste-activated sludge | MYp |
References
- Tytła, M. The effects of ultrasonic disintegration as a function of waste activated sludge characteristics and technical conditions of conducting the process—Comprehensive analysis. Int. J. Environ. Res. Public Health 2018, 15, 2311. [Google Scholar] [CrossRef] [Green Version]
- Kroiss, H. What is the potential for utilizing the resources in sludge? Water Sci. Technol. 2004, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.R.; Mehrdadi, N.; Bidhendi, G.N.; Torabian, A. Excess sludge reduction using ultrasonic waves in biological wastewater treatment. Desalination 2011, 275, 67–73. [Google Scholar] [CrossRef]
- Kuglarz, M.; Karakashev, D.; Angelidaki, I. Microwave and thermal pretreatment as methods for increasing the biogas potential of secondary sludge from municipal wastewater treatment plants. Bioresour. Technol. 2013, 134, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ekama, G.A.; Sötemann, S.W.; Wentzel, M.C. Biodegradability of activated sludge organics under anaerobic conditions. Water Res. 2007, 41, 244–252. [Google Scholar]
- Guan, R.; Yuan, X.; Wu, Z.; Jiang, L.; Li, Y.; Zeng, G. Principle and application of hydrogen peroxide based advanced oxidation processes in activated sludge treatment: A review. Chem. Eng. J. 2018, 339, 519–530. [Google Scholar] [CrossRef]
- Lee, I.; Han, J. Ultrasonics Sonochemistry the effects of waste-activated sludge pretreatment using hydrodynamic cavitation for methane production. Ultrason. Sonochem. 2013, 20, 1450–1455. [Google Scholar] [CrossRef]
- Raheem, A.; Sikarwar, V.S.; He, J.; Dastyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Garlicka, A.; Umiejewska, M.Z.-S.K.; Roubinek, O.; Palige, J.; Chmielewski, A. Effects of thickened excess sludge pre-treatment using hydrodynamic cavitation for anaerobic digestion. Energies 2020, 13, 2483. [Google Scholar] [CrossRef]
- Neumann, P.; Pesante, S.; Venegas, M.; Vidal, G. Developments in pre-treatment methods to improve anaerobic digestion of sewage sludge. Rev. Environ. Sci. Biotechnol. 2016, 15, 173–211. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Koupaie, E.H.; Johnson, T.; Eskicioglu, C. Comparison of different electricity-based thermal pretreatment methods for enhanced bioenergy production from municipal sludge. Molecules 2018, 23, 2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.K.; Shin, H.S.; Kim, D.H. Waste activated sludge hydrolysis during ultrasonication: Two-step disintegration. Bioresour. Technol. 2012, 121, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lu, Y.; Zheng, L.; Wang, Z.; Dai, X. Perspective on enhancing the anaerobic digestion of waste activated sludge. J. Hazard. Mater. 2019, 389, 121847. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeong, E.; Oh, S.E.; Shin, H.S. Combined (alkaline+ultrasonic) pretreatment effect on sewage sludge disintegration. Water Res. 2010, 44, 3093–3100. [Google Scholar] [PubMed]
- Eskicioglu, C.; Kennedy, K.J.; Droste, R.L. Characterization of soluble organic matter of waste activated sludge before and after thermal pretreatment. Water Res. 2006, 40, 3725–3736. [Google Scholar] [CrossRef]
- Salsabil, M.R.; Laurent, J.; Casellas, M.; Dagot, C. Techno-economic evaluation of thermal treatment, ozonation and sonication for the reduction of wastewater biomass volume before aerobic or anaerobic digestion. J. Hazard. Mater. 2010, 174, 323–333. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, P.; Zhang, G.; Jin, S.; Li, D.; Zhang, M.; Xu, X. Effect of alkaline addition on anaerobic sludge digestion with combined pretreatment of alkaline and high pressure homogenization. Bioresour. Technol. 2014, 168, 167–172. [Google Scholar] [CrossRef]
- Huan, L.; Yiying, J.; Mahar, R.B.; Zhiyu, W.; Yongfeng, N. Effects of ultrasonic disintegration on sludge microbial activity and dewaterability. J. Hazard. Mater. 2009, 161, 1421–1426. [Google Scholar] [CrossRef]
- Kim, D.H.; Cho, S.K.; Lee, M.K.; Kim, M.S. Increased solubilization of excess sludge does not always result in enhanced anaerobic digestion efficiency. Bioresour. Technol. 2013, 143, 660–664. [Google Scholar] [CrossRef]
- Uma Rani, R.; Adish Kumar, S.; Kaliappan, S.; Yeom, I.T.; Rajesh Banu, J. Low temperature thermo-chemical pretreatment of dairy waste activated sludge for anaerobic digestion process. Bioresour. Technol. 2012, 103, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Zhang, Z.; Pan, Y.; Tan, Y.; Lu, X.; Zhen, G. Does the combined free nitrous acid and electrochemical pretreatment increase methane productivity by provoking sludge solubilization and hydrolysis? Bioresour. Technol. 2020, 304, 123006. [Google Scholar] [CrossRef] [PubMed]
- Taboada-Santos, A.; Braz, G.H.R.; Fernandez-Gonzalez, N.; Carballa, M.; Lema, J.M. Thermal hydrolysis of sewage sludge partially removes organic micropollutants but does not enhance their anaerobic biotransformation. Sci. Total Environ. 2019, 690, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Li, Y.Y.; Zhao, Y. Combined electrical-alkali pretreatment to increase the anaerobic hydrolysis rate of waste activated sludge during anaerobic digestion. Appl. Energy 2014, 128, 93–102. [Google Scholar] [CrossRef]
- Feijoo, G.; Soto, M.; Mhdez, R.; Lema, J.M. Sodium inhibition in the anaerobic digestion process: Antagonism and adaptation phenomena. Enzym. Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Cao, S.; Sun, F.; Lu, D.; Zhou, Y. Characterization of the refractory dissolved organic matters (rDOM)in sludge alkaline fermentation liquid driven denitrification: Effect of HRT on their fate and transformation. Water Res. 2019, 159, 135–144. [Google Scholar]
- Zhu, Y.F.; Liu, H.B.; Liu, H.; Huang, S.; Ma, H.J.; Tian, Y. Filtration characteristics of anaerobic fermented sewage sludge for fatty acids production. Sep. Purif. Technol. 2015, 142, 8–13. [Google Scholar] [CrossRef]
- Mostafa, A.; Im, S.; Song, Y.C.; Kang, S.; Kim, D.H. Enhanced Anaerobic Digestion of Long Chain Fatty Acid by Adding Magnetite and Carbon Nanotubes. Microorganisms 2020, 8, 333. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Han, S.K.; Kim, S.H.; Shin, H.S. Effect of gas sparging on continuous fermentative hydrogen production. Int. J. Hydrog. Energy 2006, 31, 2158–2169. [Google Scholar] [CrossRef]
- Andrew, D.E.; American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Wang, X.; Zhang, L.; Peng, Y.; Zhang, Q.; Li, J.; Yang, S. Enhancing the digestion of waste activated sludge through nitrite addition: Insight on mechanism through profiles of extracellular polymeric substances (EPS) and microbial communities. J. Hazard. Mater. 2019, 369, 164–170. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of thermal sludge pre-treatment processes to improve dewaterability. J. Hazard. Mater. 2003, 98, 51–67. [Google Scholar] [CrossRef]
- Carrere, H.; Antonopoulou, G.; Affes, R.; Passos, F.; Battimelli, A.; Lyberatos, G.; Ferrer, I. Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresour. Technol. 2016, 199, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.J.; Tyagi, R.D.; Surampalli, R.Y. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Neis, U.; Nickel, K.; Tiehm, A. Enhancement of anaerobic sludge digestion by ultrasonic disintegration. Water Sci. Technol. 2000, 42, 73–80. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation—Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.H.; Zeng, G.M.; Wang, H.L.; Yan, J.W.; Xu, H.Y.; Gou, C.L. A novel approach for improving the drying behavior of sludge by the appropriate foaming pretreatment. Water Res. 2015, 68, 667–679. [Google Scholar]
- Bijan, L.; Mohseni, M. Integrated ozone and biotreatment of pulp mill effluent and changes in biodegradability and molecular weight distribution of organic compounds. Water Res. 2005, 39, 3763–3772. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, B.; Wang, D.; Ma, T.; Xia, H.; Yu, D. Influence of wastewater sludge treatment using combined peroxyacetic acid oxidation and inorganic coagulants re-flocculation on characteristics of extracellular polymeric substances (EPS). Water Res. 2016, 88, 728–739. [Google Scholar]
- Xin, X.; He, J.; Feng, J.; Li, L.; Wen, Z.; Hu, Q.; Qiu, W.; Zhang, J. Solubilization augmentation and bacterial community responses triggered by co-digestion of a hydrolytic enzymes blend for facilitating waste activated sludge hydrolysis process. Chem. Eng. J. 2016, 284, 979–988. [Google Scholar] [CrossRef]
- Tian, X.; Wang, C.; Trzcinski, A.P.; Lin, L.; Ng, W.J. Interpreting the synergistic effect in combined ultrasonication-ozonation sewage sludge pre-treatment. Chemosphere 2015, 140, 63–71. [Google Scholar] [CrossRef]
- Tian, X.; Wang, C.; Trzcinski, A.P.; Lin, L.; Ng, W.J. Insights on the solubilization products after combined alkaline and ultrasonic pre-treatment of sewage sludge. J. Environ. Sci. (China) 2015, 29, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abelleira-Pereira, J.M.; Pérez-Elvira, S.I.; Sánchez-Oneto, J.; de la Cruz, R.; Portela, J.R.; Nebot, E. Enhancement of methane production in mesophilic anaerobic digestion of secondary sewage sludge by advanced thermal hydrolysis pretreatment. Water Res. 2015, 71, 330–340. [Google Scholar] [PubMed]
- Ruiz-Hernando, M.; Martinez-Elorza, G.; Labanda, J.; Llorens, J. Dewaterability of sewage sludge by ultrasonic, thermal and chemical treatments. Chem. Eng. J. 2013, 230, 102–110. [Google Scholar] [CrossRef] [Green Version]


| Series of Pretreatments | DD * by Individual Pretreatment (%) | Calculated DD (%) (A = a + b + c) | Actual DD (%) (B) | Synergistic Impact (%) (C = B − A) | CH4 Yield from Total (MYt, %) | CH4 Yield from Soluble Fraction (MYs, %) | CH4 Yield from Particulate Fraction (MYp, %) | ||
|---|---|---|---|---|---|---|---|---|---|
| Alkaline (=a) | Thermal (=b) | Ultrasonic (=c) | |||||||
| (A → T) | 13.2 ± 1.0 | 12.7 ± 0.8 | - | 25.9 | 42.9 ± 2.9 | 17.0 | 68.8 ± 2.9 | 80.3 ± 4.7 | 60.2 |
| (T → A) | 13.2 ± 1.0 | 12.7 ± 0.8 | - | 25.9 | 28.3 ± 1.6 | 2.4 | 62.3 ± 3.6 | 82.4 ± 4.2 | 54.4 |
| (T → U) | - | 12.7 ± 0.8 | 12.8 ± 0.9 | 25.5 | 22.9 ± 1.4 | −2.6 | 57.6 ± 2.4 | 88.3 ± 5.7 | 48.5 |
| (U → T) | - | 12.7 ± 0.8 | 12.8 ± 0.9 | 25.5 | 27.1 ± 1.2 | 1.6 | 56.7 ± 3.3 | 94.6 ± 4.0 | 42.6 |
| (U → A) | 13.2 ± 1.0 | - | 12.8 ± 0.9 | 26.0 | 30.7 ± 1.3 | 4.7 | 57.5 ± 3.6 | 84.8 ± 5.1 | 45.4 |
| (A → U) | 13.2 ± 1.0 | - | 12.8 ± 0.9 | 26.0 | 32.0 ± 2.1 | 6.0 | 58.6 ± 3.3 | 84.2 ± 5.8 | 46.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, A.; Kim, M.-G.; Im, S.; Lee, M.-K.; Kang, S.; Kim, D.-H. Series of Combined Pretreatment Can Affect the Solubilization of Waste-Activated Sludge. Energies 2020, 13, 4165. https://0-doi-org.brum.beds.ac.uk/10.3390/en13164165
Mostafa A, Kim M-G, Im S, Lee M-K, Kang S, Kim D-H. Series of Combined Pretreatment Can Affect the Solubilization of Waste-Activated Sludge. Energies. 2020; 13(16):4165. https://0-doi-org.brum.beds.ac.uk/10.3390/en13164165
Chicago/Turabian StyleMostafa, Alsayed, Min-Gyun Kim, Seongwon Im, Mo-Kwon Lee, Seoktae Kang, and Dong-Hoon Kim. 2020. "Series of Combined Pretreatment Can Affect the Solubilization of Waste-Activated Sludge" Energies 13, no. 16: 4165. https://0-doi-org.brum.beds.ac.uk/10.3390/en13164165